Reports: UNI152943-UNI1: Foldameric Catalysts for Enantioselective Trifluoromethylations of Ketones and Enones
Benjamin Gorske, PhD, Bowdoin College
The incorporation of the trifluoromethyl group into petroleum-derived carbonyl compounds is critical in the design and manufacture of many pharmaceuticals, polymers, and agrochemicals. However, the development of general and enantioselective methods for nucleophilic trifluoromethylations has been hindered by the difficulty of developing existing organocatalyst scaffolds in a rapid, parallel fashion. Peptoid (N-alkyl glycine oligomer) organocatalysts are superbly suited to address this challenge because they can be rapidly synthesized to generate combinatorial libraries of catalysts. Moreover, the large size of peptoids relative to many other organocatalysts could facilitate multiple contacts between substrates and catalytic functional groups, engendering bifunctional catalysis that might activate less reactive substrates. Perhaps most importantly, the folding of peptoids can be dictated by weak noncovalent interactions that could pre-organize, but not necessarily rigidify, a peptoid catalyst. Such a catalyst might accommodate an expanded set of substrates and actualize a generally useful route to enantiopure trifluoromethylated alcohols and ketones. Therefore, this proposal aims to develop peptoid catalysts that will accomplish enantioselective trifluoromethylations of ketones and enones efficiently, enantioselectively, and with broad scope. These catalysts will provide rapid access to trifluoromethylated subunits of pesticides and herbicides, polymeric materials, and potent anti-cancer, antiviral, and antimicrobial agents.
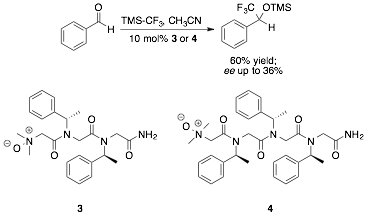
As detailed in last year's report, we discovered that acetonitrile is a highly effective solvent for nucleophilic trifluoromethylation – an important development given that peptoid secondary structure is well understood in this solvent. We were also gratified to observe that, while the ee observed for dimer 3 was quite low, an ee of 36% was obtained using trimeric catalyst 4 (Figure 1).
Figure 1. Initial tests of catalyst enantioselectivity in the nucleophilic trifluoromethylation of benzaldehyde.
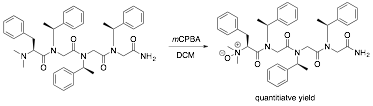
Given the successes of these preliminary experiments, we focused our efforts on scaling up the syntheses of the catalysts and improving our purification protocols. However, our attempts to reproduce oxidations of peptoids to the corresponding N-oxide catalysts using the BAP reagent failed. Subsequent experiments revealed that the N,N-dimethyl-N-oxide group can be displaced by nucleophiles under neutral and acidic conditions. After alternative functional groups such as tertiary amines and carboxylates failed to exhibit sufficient catalytic activity, we discovered that incorporation of N,N-dimethylphenylalanine as the precatalytic residue blocked this reaction pathway and, in conjunction with oxidation using meta-chloroperbenzoic acid (mCPBA), generated the desired catalyst in quantitative yield (Figure 2). This methodology was used to successfully synthesize a series of peptoid catalysts analogous to 4 but varying in length from three to six residues. In addition, catalysts containing hydrogen bond donors were also constructed in an effort to engender bifunctional catalysis that could dramatically enhance performance. Assessment of the catalytic activities of these compounds is ongoing in my laboratory.
Figure 2. Oxidation of peptoids giving quantitative yields of putative nucleophilic trifluoromethylation catalysts.
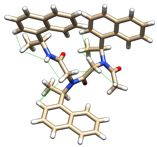
In parallel with the work above, we also sought to determine the catalytic potential of peptoids containing both D- and L-residues, as analogous peptides have proven to be exceptionally powerful and versatile catalyst scaffolds. To date, several studies have shown that mixing side chain stereoconfigurations within a peptoid decreases its conformational stability and homogeneity, likely due to the suppression of folding cooperativity. We reasoned that 1-naphthylethyl groups in particular might stabilize new peptoid structures in the absence of cooperative folding due to their extended reach, which could facilitate contact with distant atoms and thereby restrict not only the w dihedral angle of the nearby amide, but also the f, y, and nearby sidechain dihedral angles as well. This design scheme allowed us to realize several new and unique peptoid structures with high conformational homogeneity, including a 'w-strand' motif for which we were able to obtain the first atomic resolution X-ray crystal structures (Figure 3). These previously inaccessible secondary structures constitute an entirely new class of discretely folded peptoids with potential applications in not only catalysis, but also materials design and biochemical investigation. Examination of the catalytic potential of these motifs is currently underway in my laboratory.
Figure 3. X-ray crystal structure of a new peptoid 'w-strand' motif, with nOes observed in solution shown as green lines.
The support provided by this grant has allowed me to lay the foundations for a successful research program that has already yielded a significant publication. Moreover, the program has excited and inspired undergraduates to join me in pursuing this research, which in turn has inspired several of them to pursue chemistry degrees and careers in the sciences. Going forward, I envision applying the synthetic methodologies we are developing towards the synthesis of large libraries of peptoid catalysts useful for not only trifluoromethylations, but also for other classes of reactions that are difficult to conduct using typical small-molecule catalysts. These pursuits could ultimately open avenues toward new and efficient syntheses of petroleum-derived products.