Reports: DNI1053874-DNI10: Enhancing Oxidation Catalysis with Ti-Based Metal Organic Frameworks by Tuning Structure and Composition
Jason Hicks, PhD, University of Notre Dame
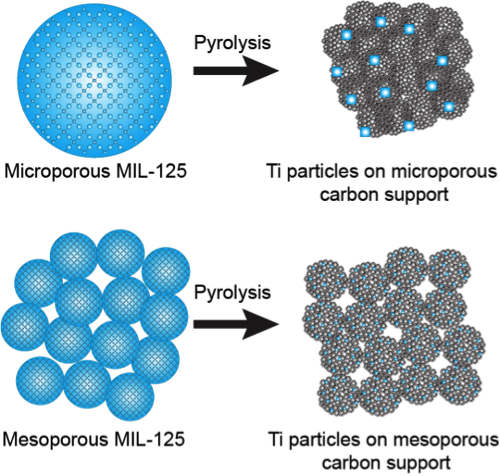
In our previous work, we detailed a method of preparing carbon-supported metal catalysts through the use of metal-organic frameworks (MOFs) as templates. In this method, organometallic compounds (such as titanium isopropoxide) were coordinated to the pendant –NH2 functionalities present on a Zn-based MOF (IRMOF-3). These postsynthetically modified MOFs were then pyrolyzed at temperatures exceeding the boiling point of Zn (> 908 C) to produce a Zn-free carbon support containing nanoparticles of the desire metal (Ti, in this case). These materials worked quite well as catalysts for sulfur oxidation reactions. However, they suffered from low metal loadings (< 5 wt. % Ti). Furthermore, the resulting Ti metal phase could not be controlled due to the high pyrolysis temperatures necessary for the elimination of Zn. Pyrolysis of the Ti-based MOF MIL-125 would avoid these problems, however, the presence of Zn during pyrolysis is advantageous because its carbothermal reduction and evaporation leads to significant mesopore formation. Therefore, we have first synthesized a mesoporous analogue of MIL-125 (as detailed in our previous progress report) and subjected this material to pyrolysis at various temperatures in order to synthesize a class of mesoporous carbon-supports containing a high loading of Ti nanoparticles (Figure 1). As a control, we also pyrolyzed microporous MIL-125 at a series of pyrolysis temperatures as well. The resulting class of materials were given the abbreviations of either micro-XXX or meso-XXX wherein micro/meso denotes the type of MIL-125 precursor used and XXX refers to the pyrolysis temperature (i.e. micro-800 denotes a material synthesized by pyrolysis microporous MIL-125 at 800 C).
Figure 1. Pyrolysis of microporous and mesoporous MIL-125 analogues to form a class of carbon-supported Ti nanoparticle catalysts.
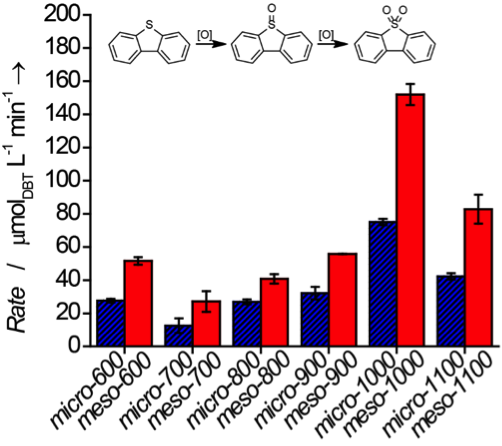
These newly carbon-supported Ti catalysts were first extensively characterized using an array of techniques. TGA experiments showed that these resulting materials contained high loadings of Ti (> 40 wt. %). Additionally, N2 physisorption analysis revealed that the meso-XXX materials always contained a more significant amount of mesoporosity compared to the micro-XXX materials synthesized under identical conditions. These materials were then tested as a catalysts in the oxidation of dibenzothiophene (DBT) by tert-butyl hydroperoxide (TBHP). Catalysis data (Figure 2) was fit to pseudo-first-order kinetics and it was discovered the meso-XXX materials exhibited faster reaction rates compared to the micro-XXX materials. These characterization and catalysis results implied that the enhancement in reaction rate of the meso-XXX materials over the micro-XXX materials was due to greater accessibility of DBT to catalytically active sites through the incorporation of mesoporosity.
Figure 2. Initial reaction rates of the oxidation of DBT by TBHP by the (blue, patterned) micro-XXX materials and the (red, filled) meso-XXX materials.
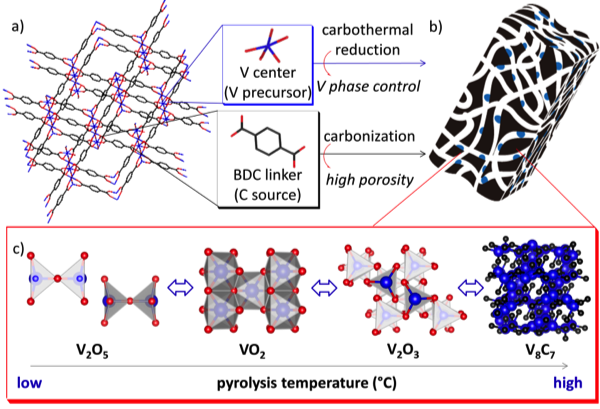
In a parallel series of work, we discovered that the V-based MOF MIL-47 was very active as a catalyst for sulfur oxidation reactions. Unfortunately, this material was extremely unstable under typical catalysis conditions and could not be recycled for repeated use. The MIL-47 structure was lost during catalysis and resulted in the formation of vanadium oxide. Carbon-supported catalysts, on the other hand, tend to exhibit high chemical and thermal stabilities. In an effort to retain the high activity inherent to MIL-47 while imparting stability, MIL-47 was subjected to pyrolysis at temperatures from 600 to 1100 C to form carbon-supported nanoparticles of different phases of V (Figure 3). These materials were given the abbreviations of CXXX where C- represents carbon and XXX represents the pyrolysis temperature (i.e. C1100 represents MIL-47 pyrolyzed at 1100 C).
Figure 3. Schematic representation of a) MIL-47 (V) pyrolyzed to form b) carbon supporting V nanoparticles with c) variable phases.
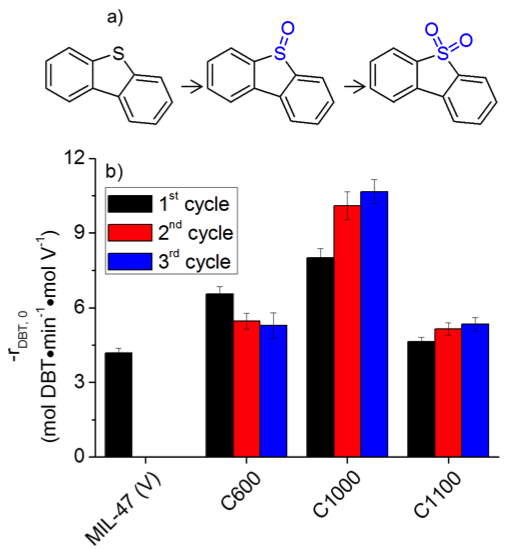
The resulting materials (C600, C1000, and C1100) were characterized and utilized for catalysis (Figure 4). Characterization techniques revealed that C600 was composed of carbon-supported V oxide particles. C1000 and C1100, however, were composed of carbon-supported V oxide and V carbide particles. The V oxide material (C600) showed a significant leaching of highly active V species (V4+ and V5+) during each reaction run. The V carbide catalysts (C1000 and C1100), on the other hand, exhibited a reduced amount of leaching in conjunction with relatively high activities in the oxidation of sulfur compounds. Finally, the C1100 material was compared to V supported on carbon prepared via the conventional incipient wetness technique. It was found that C1100 outperformed this conventional material both in terms of higher activity and reduced leaching. These studies effectively demonstrated the advantages of using the MOF-templated synthetic technique to prepare oxidative desulfurization catalysts.
Figure 4. a) Scheme for the oxidation of dibenzothiophene. b) Initial reaction rates (-rDBT,0) for the oxidation of DBT with TBHP using the pyrolyzed MIL-47 materials as catalysts for multiple consecutive reactions.