Reports: ND353839-ND3: Dinitrogen Reduction by Pincer Complexes of Early Transition Metals
Gregory S. Girolami, PhD, University of Illinois (Urbana-Champaign)
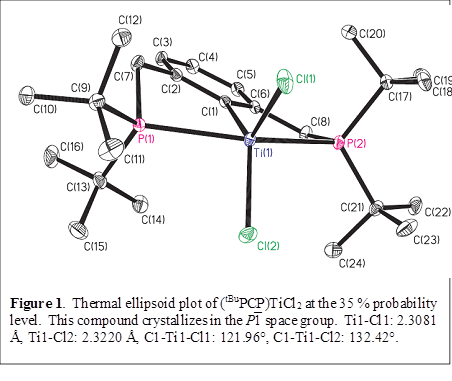
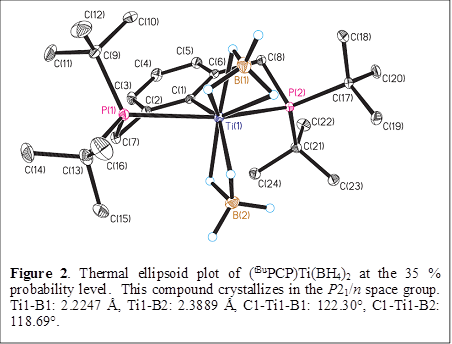
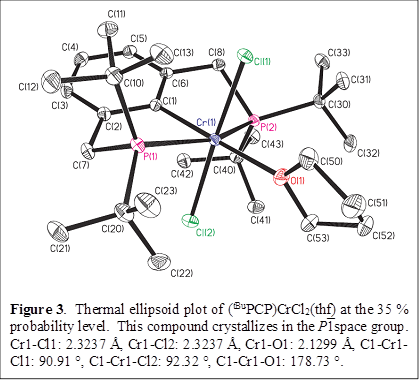
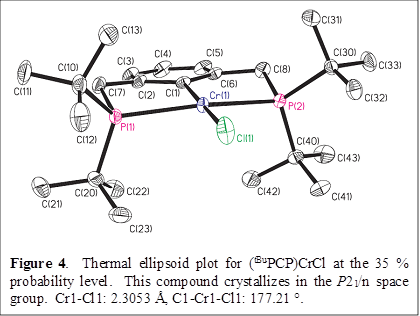
Gregory S. Girolami, PhD, University of Illinois (Urbana-Champaign)




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society