Reports: ND353621-ND3: Redox Robust Non-Noble Metal Catalysis
Jeffery Allen Byers, PhD, Boston College
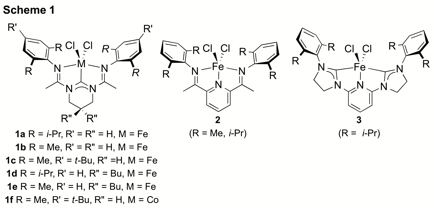
During this funding period, we have continued our investigations into the bonding and catalytic properties of bis(amindinato)-N-heterocyclic carbene iron and cobalt complexes (e.g. 1, Scheme 1), which are structural analogs to the catalytically prolific bis(imino)pyridine and bis(N-heterocyclic carbeno)pyridine iron and cobalt complexes (e.g. 2 and 3, respectively, Scheme 1){ JACS 1999, 121, 8728; JACS 1998, 120, 4049; JACS 2006, 128, 13340; Science 2012, 335, 567; Science 2015, 349, 960)}. In addition, we have continued our investigations into iron-catalyzed cross coupling reactions using iron complexes. These efforts have moved away from the bis(amino)pyridine complexes (and its analogs) and have led to investigations towards the development of iron-catalyzed alkyl-aryl and alkyl-alkyl Suzuki cross coupling reactions.
In the previous term, we outlined our studies into the electronic structure of bis(amidinato)-N-heterocyclic carbene iron complexes. We have since published the data pertaining to iron(II) and iron(III) complexes {Dalton Trans 2015, 44, 16703}, and have initiated a collaboration with Michael Neidig at the University of Rochester that is aimed towards understanding the unusual bonding features of this NHC complex in the broader context of iron complexes containing pincer ligands. A publication in a high quality journal is expected from this work by the end of the calendar year.
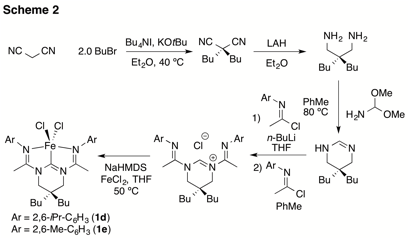
In addition to this fundamental work, we also investigated the reactivity of reduced forms of 1 towards alkene hydrogenation. A brief investigation involving the in situ activation of 1a and 1b with Na/Hg amalgam in tetrahydrofuran (THF) led to modest activity for the hydrogenation of alkenes. We hypothesized that the low activity was due to competitive binding of THF and the substrate to the metal center. To address this issue, we synthesized more soluble complexes 1c-1e (Scheme 2) as well as the cobalt complex 1f. These complexes proved to be significantly more soluble in hydrocarbon solvents and, as predicted, significantly more active for the hydrogenation of alkenes.
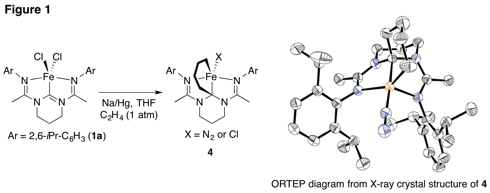
To further increase reactivity, we are now working towards isolating the reduced iron complexes containing dinitrogen ligands, which the Chirik group has shown to be particularly good catalysts for alkene hydrogenation. As part of this ongoing effort, we have discovered that the bis(amidinato)-N-heterocyclic carbene have demonstrated some unique reactivity. For example, reducing 1a in the presence of ethylene without hydrogen led to the isolation of metallocyclohexane 4 (Figure 1). This reaction features involvement of the N-heterocyclic carbene carbon, which highlights the difference between 1 and 3 and may be an important feature of catalytic reactions involving bis(amidinato)-N-heterocyclic carbene complexes. A manuscript describing these efforts is in preparation and will also be published this winter.
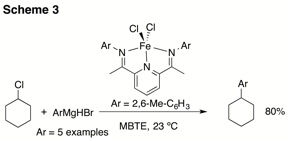
In a parallel effort, we had begun to investigate the catalytic cross coupling of alkyl halides with Grignard's utilizing bis(imino)pyridine complexes 2 as catalysts (Scheme 3). While these complexes were excellent catalysts for coupling cyclohexyl chloride with various Grignard nucleophiles, the reaction had a limited scope with respect to the electrophile.
This led to a significant shift in direction for this project, which is now focused on developing iron-catalyzed aryl-alkyl and alkyl-alkyl Suzuki reactions. While there are several examples of iron-catalyzed Kumada-type cross coupling reactions {Chem. Rev. 2011, 111, 1417}, there are very few iron-catalyzed Suzuki cross coupling reactions {Chem. Eur. J. 2014, 20, 7935; Chem. Lett. 2014, 44, 486}, and these require boronate electrophiles derived from Grignard reagents. It is believed that slow transmetallation rates are problematic for iron-catalyzed Suzuki cross coupling reactions{Organometallics 2014, 33, 370; ACS Catalysis 2012, 2, 1829}. To address this knowledge gap, we are now investigating new boron-containing electrophiles for catalytic cross coupling reactions that are expected to be more efficient in transmetalation reactions as well as discrete intermediates that are believed to be involved in the transmetalation process. Stoichiometric and catalytic reactions are being pursued to address this limitation in iron-catalyzed cross coupling reactions. This work was begun using the support from the PRF-DNI, and it will remain a major effort in the lab as it moves forward.
Support from the PRF-DNI award has been enormously helpful in establishing the research in the Byers group. At various times it has partially supported four different graduate students and partial support for a postdoctoral scholar. The older students who worked on the project have published articles in well-respected journals and the younger students have established a research direction that will be amenable towards obtaining their Ph. D. In terms of the impact that it has had on the research in the Byers group, support from the ACS-PRF was instrumental in establishing fundamental organometallic research in the lab that is now blossoming into useful organometallic catalysis.