Reports: UR454658-UR4: Interaction of Aromatic Hydrocarbons with Self-Assembled Cubic Supramolecular Metallocages
John D. Thoburn, PhD, Randolph-Macon College
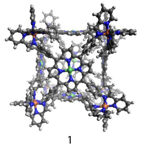
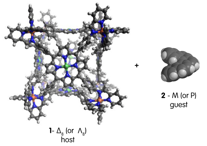
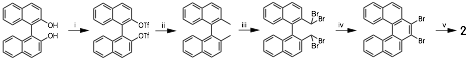
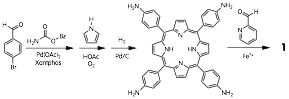
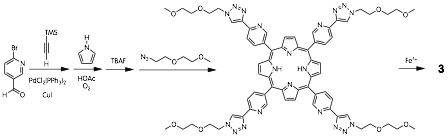
John D. Thoburn, PhD, Randolph-Macon College





Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society