Reports: DNI952190-DNI9: Linking Fractional Free Volume to Gas Solubility and Membrane Permeability in Ionic Liquids
Jason E. Bara, PhD, University of Alabama (Tuscaloosa)
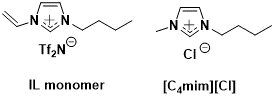
In the past year, we have made some new membrane materials based on poly(IL) materials containing metal salts (CuCl) in an effort to facilitate the transport of carbon monoxide (CO) relative to hydrogen (H2). This is an important consideration as related to syngas processing and for applications such as the water-gas shift reaction. Poly(IL) membranes were produced from an vinyl-functionalized IL monomer and either of the ILs [C4mim][Tf2N] or [C4mim][Cl]. For those materials in which [C4mim][Cl] was used, 1 equivalent of copper (I) chloride was also added, which resulted in the formation of the chlorocuprate ([CuCl2]-) anion, which was expected to catalyze transport of CO through the poly(IL) membrane material.
However, at 20oC, no facilitation of CO was observed based on the relative permeability of membranes with and without [CuCl2]- present. Table 1 shows that relative to tests with pure N2 as a control, N2/CO mixed gas showed virtually no difference in permeability. Since N2 and CO have the same MW (28 g/mol) and are approximately the same size and shape, they are expected to behave similarly in polymer membranes, except that CO is able to reversibly bind to the [CuCl2]- anion. If facilitation of CO were occurring, then the permeability of the mixed gas would be expected to increase due to more rapid transport of CO.
Although the results of this work did not produce reactive membrane materials, it did demonstrate that transition metal salts can easily be included within poly(IL) membranes which may also open opportunities for other species known to facilitate transport of gases such as Co2+ which is known to show a reversible binding to O2.
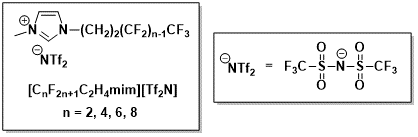
Over the past several years, I have also had the opportunity to collaborate with the research group of Prof. Sergey Verevkin in Germany to measure the vaporization enthalpies of ILs in order to obtain thermodynamic data that can help my research to understand the molecular-level behavior of ILs so as to better tailor them for membrane applications. This collaboration is synergistic as my group has produced a number of ILs throughout the course of the PRF-DNI award and after we are done with these samples in my lab, we are able to share some of the residual ILs with Prof. Verevkin’s group to enable their measurements. In our most recent work, we have examined the vaporization enthalpies of ILs bearing fluorinated substituents appended to the imidazolium cation. Such compounds have been exceedingly rare in the literature even though fluorinated compounds play important roles in many applications (e.g. refrigeration).
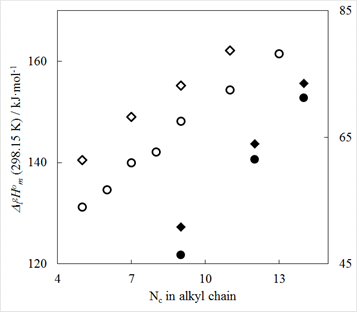
This work demonstrated that fluorinating the side chain of imidazolium-based ILs can increase the enthalpy of vaporization by ~10 kJ/mol compared to those with n-alkyl chains (Figure 3), and that the magnitude of this increase is larger than that observed for simple 1-fluoroalkane/alkane systems.
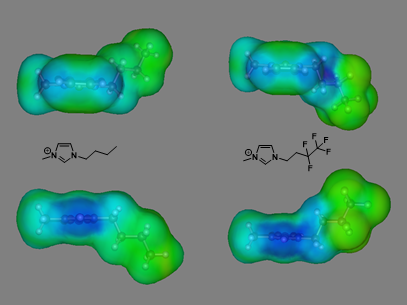
Figure 4 provides an intuitive visual comparison of the s-surfaces of the optimized structures of analogous [C4mim] and [(CF)2C2mim] cations as generated using COSMOTherm.
As can be clearly seen in Figure 4, the s-surface of the [(CF)2C2mim] cation (right) presents a larger proportional area of blue surface around the C(2), C(4) and C(5) protons compared to the [C4mim] cation (left). For the [(CF)2C2mim] cation, the electron-poor character also extends much more prevalently onto the -(CH2)- linkages of the fluorinated side chain, which is not observed for the strictly hydrocarbon side chain. This is indicative of a more electron-poor character associated with the imidazolium ring and is driven by the electron-withdrawing fluorine atoms that are located well away from the ring. This simulation, prior MD simulations, and the 1H NMR data all suggest that stronger cation-anion interactions are present in fluoroalkyl-functionalized ILs and this greater association leads to an increase in vaporization enthalpy. This further validates our other studies that show fluorinated ILs have much smaller free volumes than alkyl-containing ILs of similar size/length.
The ACS-PRF DNI program has benefitted me greatly in my career thus far. I have been able to explore some very important aspects of ionic liquids from both experimental and computational/modeling approaches. My group has been able to publish several key papers that will serve as a foundation for future work for years to come. The award has also enabled me to attend two international conferences related to ionic liquids, COIL-5 (Portugal, 2013) and IL-SEPT2 (Canada, 2014). At these meetings, I was able to interact with many well-known researchers from around the world and foster collaborations. The DNI award helped support a Ph.D. student, W. Jeffrey Horne, who graduated in August 2015. From August-December 2015, a visiting graduate student from Spain, Gabriel Zarca Lago, also actively participated in this project. Furthermore, this award has enabled me to work with a number of very talented undergraduate students, several of whom are named as co-authors on publications that have resulted from this funding.
Figure 1: Structure of IL monomer and IL used in this work.
Table 1: Gas permeability results for poly(IL) membranes studied.
|
Membrane Composition (mole %) Monomer:[C4mim][Cl]:[CuCl] |
Gas |
P (barrer) |
|
75:25:0 |
N2 |
0.9 |
|
H2 |
9.9 | |
|
N2/CO mix |
1.0 | |
|
70:25:5 |
N2 |
0.35 |
|
H2 |
5.7 | |
|
N2/CO mix |
0.34 | |
|
60:32:8 |
N2 |
0.25 |
|
H2 |
7.0 | |
|
N2/CO mix |
0.25 | |
|
50:40:10 |
N2 |
0.89 |
|
H2 |
8.2 | |
|
N2/CO mix |
0.80 |
Figure 2: Chemical structures of [(CF)nC2mim][NTf2] ILs with fluorinated substituents.
Figure 3: Comparison of vaporization enthalpies for
fluoroalkyl-substituted ILs [(CF)nC2mim][NTf2]
and with the homomorph series [Cnmim][NTf2], where NC
is the total number of all C-containing units present in the imidazolium cation
in the 1 and 3 positions, as well as comparison of vaporization enthalpies of
n-alkanes and 1-fluoro n-alkanes.○- is series [Cnmim][NTf2],
![]() is
series of [(CF)nC2mim][NTf2], ● is
series of n-alkanes, and ♦
is series of 1-fluoro n-alkanes. The primary (left) y-axis is for ILs
and the secondary (right) y-axis is for alkanes (vaporization enthalpy values
were scaled for the sake of presentation).
is
series of [(CF)nC2mim][NTf2], ● is
series of n-alkanes, and ♦
is series of 1-fluoro n-alkanes. The primary (left) y-axis is for ILs
and the secondary (right) y-axis is for alkanes (vaporization enthalpy values
were scaled for the sake of presentation).
Figure 4: s-surfaces of the [C4mim] cation (left) and [(CF)2C2mim] cation (right). The top views present the edge-on surface surrounding the C(4) and C(5) protons and the bottom views present the edge-on surface surrounding the C(2) proton.