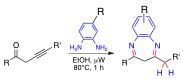Reports: UR152858-UR1: Effective Catalyzed and Electrophilic Cyclization Reactions Leading to Highly and Diversely Substituted Fluoroheterocycles
Roman Dembinski, Oakland University
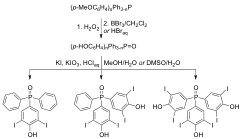
In separate efforts, we were attracted to the synthesis of 3,4,5-substituted triaryl phosphines oxides containing groups that offer a starting point for further synthetic transformations. Our attention focused on the 4-hydroxyphenyl substituent, which can be converted into various ligands including perfluoroalkyl (fluorous) chains. The 3,5-diiodomotif has also been synthetically followed for applications such as the construction of organometallic units. Combining, the synthesis of diversely trisubstituted phosphine oxides containing thyroxine-related 4-hydroxy-3,5-diiodophenyl fragments has been approached. Accordingly (4-hydroxy-3,5-diiodophenyl)diphenylphosphine oxide, bis(4-hydroxy-3,5-diiodophenyl)(phenyl)phosphine oxide, and tris(4-hydroxy-3,5-diiodophenyl)phosphine oxide were obtained via iodination reactions of corresponding mono-, bis-, and tris(4-hydroxyphenyl)phosphine oxides. Single-crystal X-ray diffraction studies showed each homologue forms O-H•••O=P and pi-stacking interactions with neighboring molecules to give inversion related dimeric motifs.
This year three undergraduate students were involved in this project and two enjoyed a summer research opportunity (the third went to Germany for another summer program). All students are remaining in the PI's laboratory, while pursuing their B.S. in Chemistry (ACS accredited).