Reports: DNI153428-DNI1: Bronsted Acid-Catalyzed Reactions of Unsaturated Acetals
Pavel Nagorny, Ph.D., University of Michigan
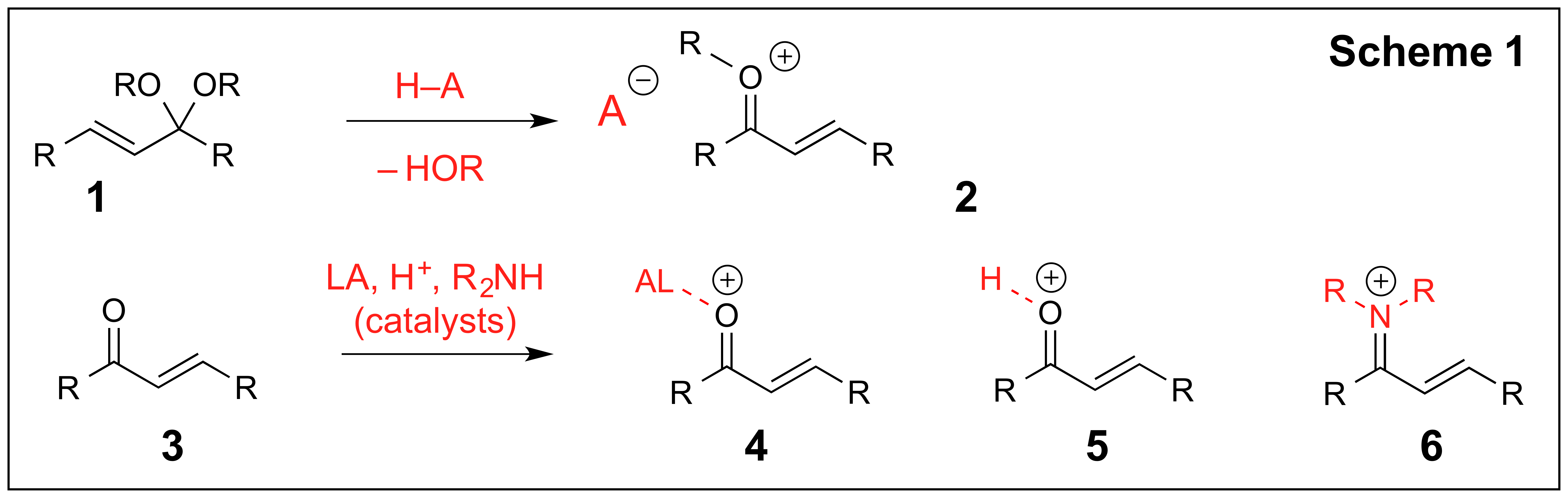
The long-term objective of this study is to develop new organocatalytic transformations of a,b-unsaturated acetals (1, Scheme 1). Br∅nsted acid-catalyzed reactions of a,b-unsaturated acetals have been extensively used in organic synthesis for a variety of different transformations including [2+4], [3+4] and [5+2] cycloadditions, Nazarov cyclizations and conjugate additions.
The reactivity of a,b-unsaturated acetals (1) often parallels the reactivity of a,b-unsaturated carbonyls as such reactions are often proposed to proceed through unsaturated oxocarbenium ions (2). Such intermediates are isoelectronic to the intermediates 4-6 obtained through the activation of a,b-unsaturated carbonyls via complexation with Lewis or Br∅nsted acids or the formation of unsaturated iminium ions. However, unlike the a,b-unsaturated carbonyls, it is significantly more challenging to control the reactivity of a,b-unsaturated acetals using simple organic and inorganic molecules as catalysts. Our studies are designed to address some of the existing challenges by exploring chiral hydrogen bond donors such as chiral phosphoric acids. In addition, we recently have identified thiophosphoramides as the promising organic catalysts for counterion activation, and in addition to the studies in Scheme 1 our objectives include exploration of thiophosphoramides in counterion catalysis. The results of these studies obtained over the last year are described below:
1)
 Exploring thiophosphoramide-based Hydrogen
Bond Donors (HBD) as the co-catalysts for Cu(II)-catalyzed
reactions of diaryliodonium salts with nucleophiles
Exploring thiophosphoramide-based Hydrogen
Bond Donors (HBD) as the co-catalysts for Cu(II)-catalyzed
reactions of diaryliodonium salts with nucleophiles
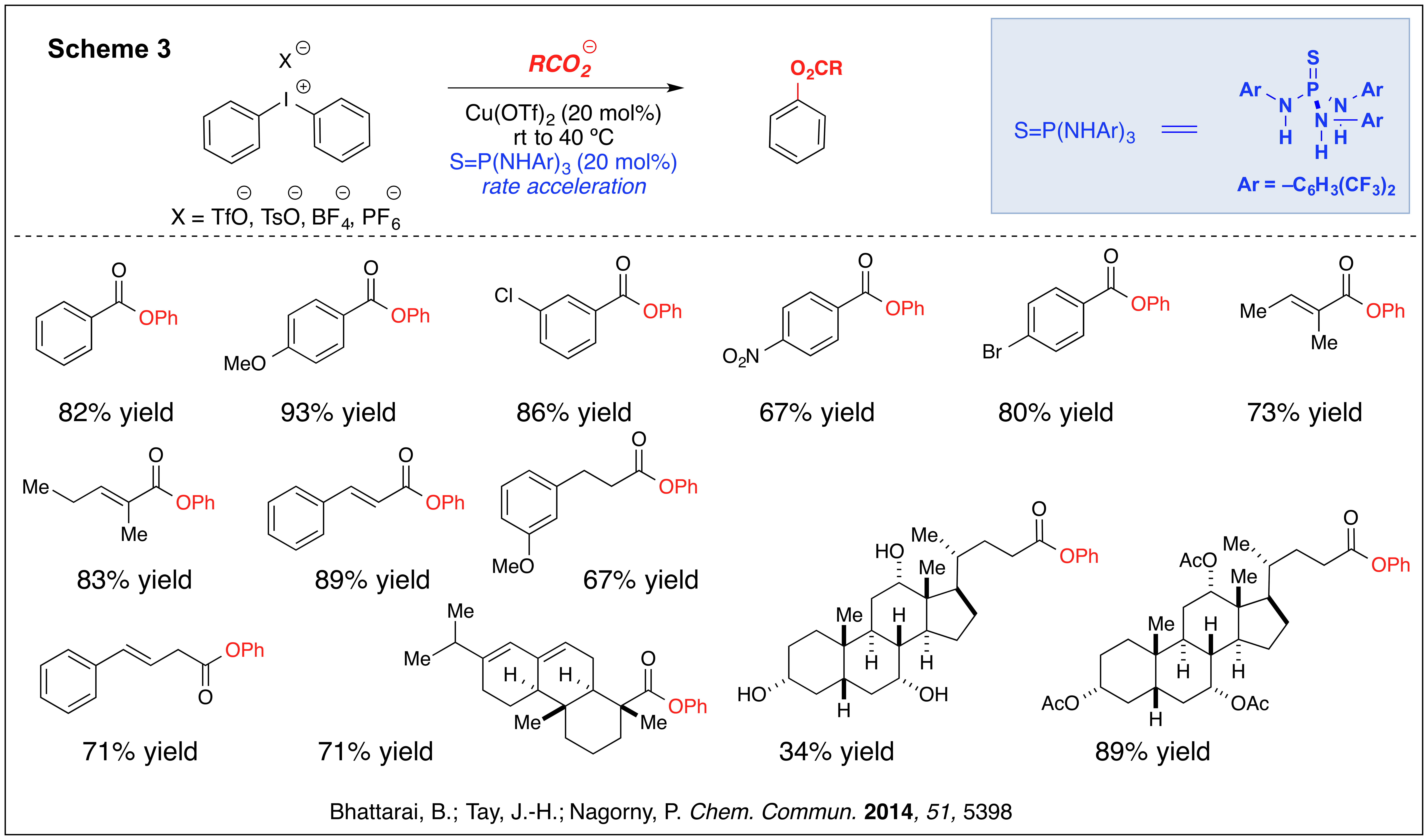
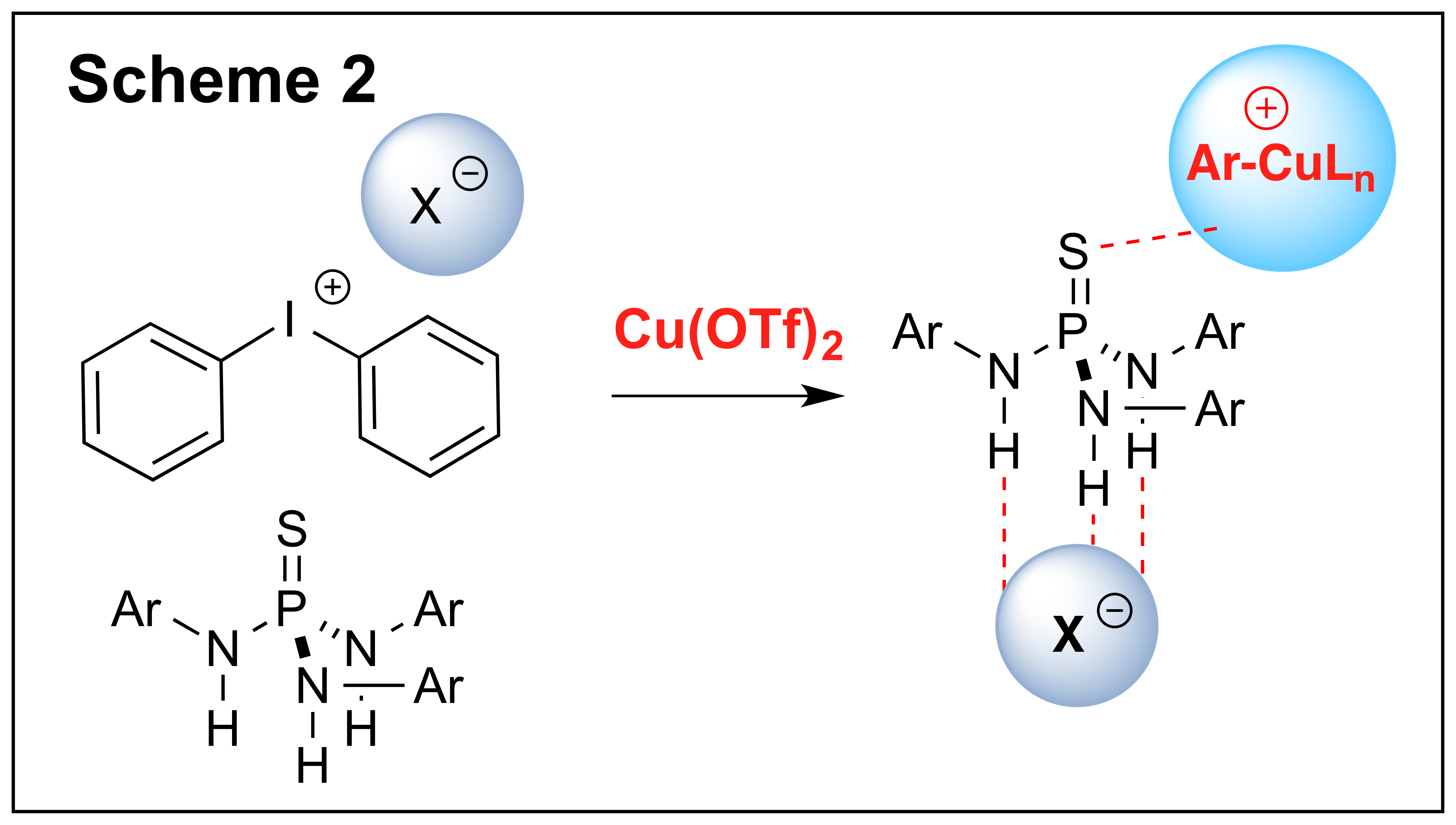
In the prior reporting cycle our group identified thiophosphoramides as the powerful co-catalysts for the Br∅nsted acid-catalyzed ionic [2+4] cycloaddition reactions involving a,b-unsaturated acetals (1). We continued investigating thiophosphoramides as the catalysts in some other transformations that can potentially benefit from counterion activation. The reaction of iodonium salts with nucleophiles that can potentially be accelerated by counterion activation depicted in Scheme 2 has been identified as one of such transformations. These reactions could proceed under both metal-catalyzed and metal-free conditions, and such parameters as the polarity of the solvent and the coordinating ability of diaryliodonium counterion are often important for the outcome of these reactions. In some instances the observed reactivity trends could be attributed to the ion-pairing effects, and certain parallels could be drawn between the reactivity of diaryliodonium salts and classical electrophiles such as iminium or carbenium ions. Our group has investigated the possibility of investigating the reactions of iodonium salts using thiophosphoramides, and have found that in combination with Cu(II) salts thiophosphoramides significantly accelerated the arylation of carboxylates (Scheme 2). As a result, the formation of a variety of phenol esters was carried at ambient temperatures with acid sensitive substrates, and the resultant aryl esters were obtained in good to excellent yields. This study was recently disclosed (Chem. Commun. 2014, 51, 5398), and our future work will focus on identifying other transformations of diaryliodonium salts that can be catalysed by thiophosphoramides.
2) Chiral Br∅nsted acid-catalyzed enantioselective intramolecular aza-Michael reaction of a,b-unsaturated acetals.
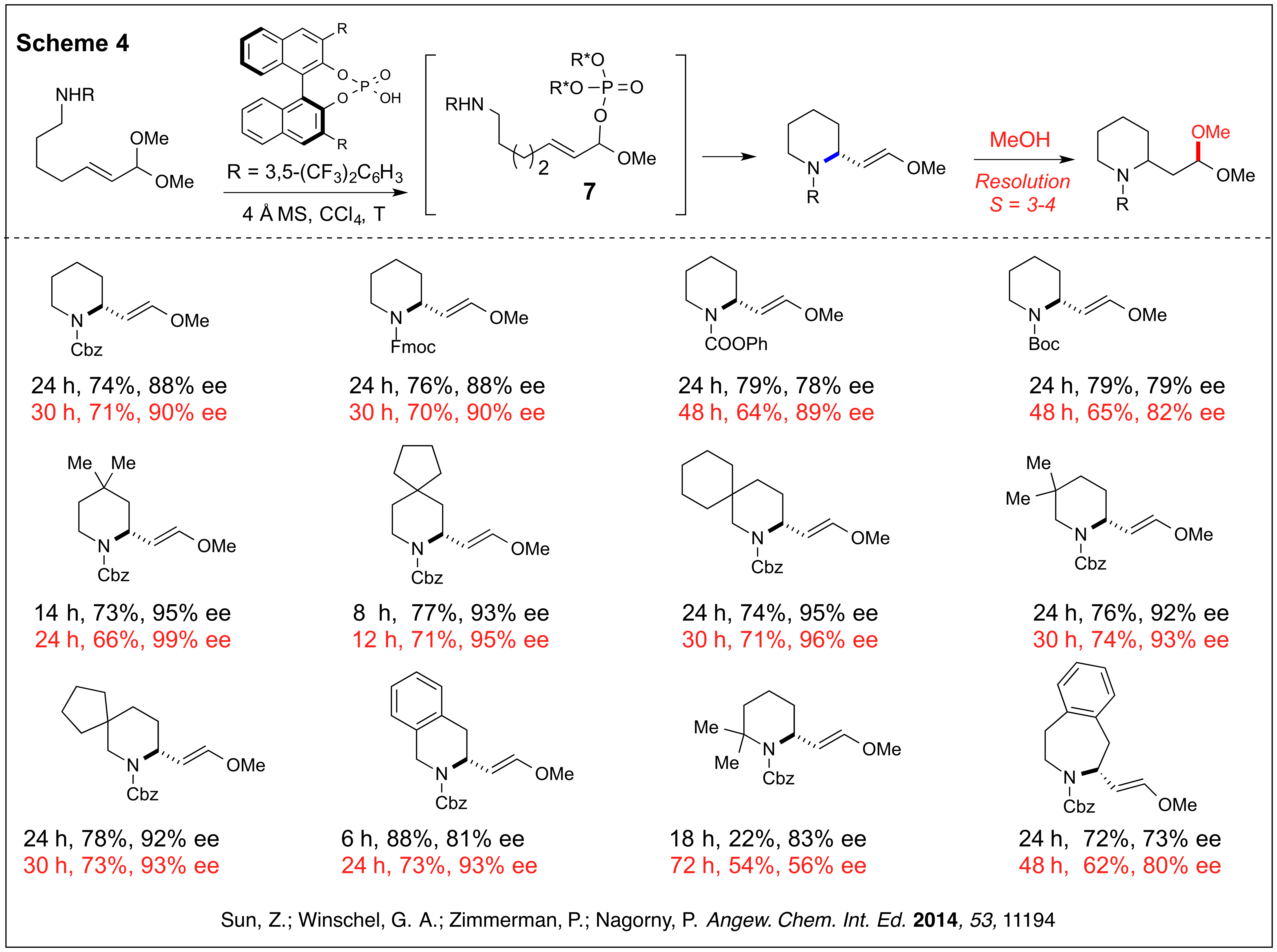
Another objective of these studies is to develop asymmetric catalytic transformations proceeding through unsaturated oxocarbenium ions 2 (Scheme 1). The high reactivity of such species coupled with their reduced Lewis basicity renders controlling the enantioselective reactions of 2 by chiral catalysts a great challenge. As a result, only auxiliary-based approaches for the reactions of a,b-unsaturated acetals proceeding through 2 existed prior to our studies. Our approach relied on the use of chiral Br∅nsted acids as the catalysts that would promote ionization of a,b-unsaturated acetals and produce a chiral ion pair 2. The chiral anion would then control the selectivity of the unsaturated oxocarbenium ion in cycloadditions or the additions of nucleophiles. Earlier, we demonstrated the validity of this for the intramolecular [2+4] cycloaddition reaction. Our following work was focused on identifying other transformation of unsaturated acetals that can be controlled by chiral phosphoric acids. The aza-Michael reaction depicted in Scheme 4 was investigated in one of our studies. Thus, we discovered that CPAs can indeed promote highly selective cyclizations leading to chiral piperidines. Remarkably, the enantioselectivities of these reactions were improved with time, which we attribute to the further resolution of the products with methanol. The computational investigation of this reaction resulted in a revised mechanistic proposal, in which a neutral anomeric phosphate 7, rather than originally proposed oxocarbenium ion pair 2 is formed as an intermediate. A manuscript describing this study has been recently published (Angew. Chem. Int. Ed. 2014, 53, 11194).
Impact:
During the second year of ACS-PRF support, the training of one postdoctoral fellow was supported by this grant. This postdoctoral fellow and coworkers have made great progress on the described projects and have generated preliminary results, materials, and spectra, which will serve as a foundation for a variety of other related projects in our group. This will also allow us to pursue new directions in HBD and Br∅nsted acid catalysis. Some of the aforementioned results were communicated in Angew. Chem. Int. Ed. and Chem. Commun.