Reports: ND1052588-ND10: Elucidating Competing Transport and Kinetic Mechanisms for Understanding Material Durability of Carbon Felt Electrodes
Venkat R. Subramanian, Washington University in St. Louis
![]()
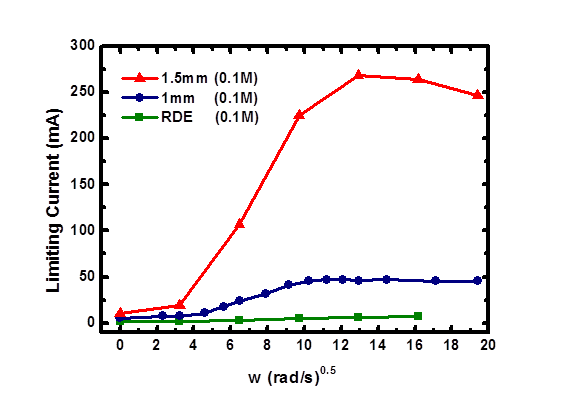
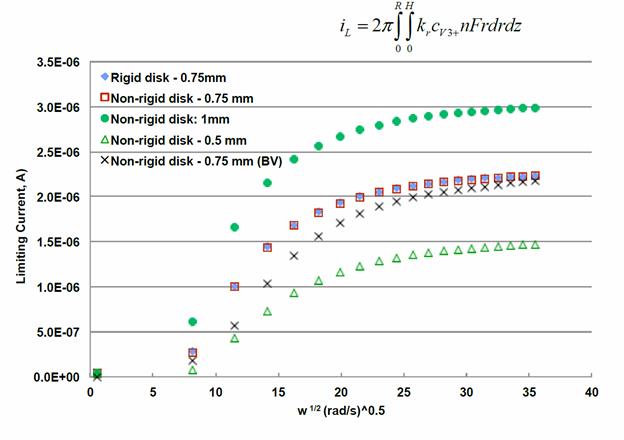
Venkat R. Subramanian, Washington University in St. Louis
![]()


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society