Reports: DNI154007-DNI1: Development of a Titanium- or Zirconium-Oxo Catalyzed Alkyne-Aldehyde Coupling Reaction for the Synthesis of Alpha,Beta-Unsaturated Carbonyls
Kami L. Hull, PhD, University of Illinois, Urbana-Champaign
Ti=O and Zr=O Mediated Alkyne-Aldehyde Coupling:
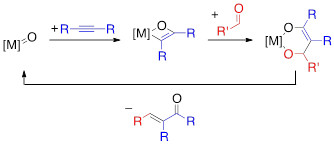
α,β-Unsaturated aldehydes and ketones are both prominent functionalities in and synthetic precursors for pharmaceuticals, natural products, and organic materials. Olefination reactions are powerful methodologies for the synthesis of enones; unfortunately, these reactions often require several synthetic steps to access the requisite starting materials and generate stoichiometric waste. The goal of the proposed research is to develop a new transition metal-catalyzed cross-coupling reaction for the synthesis of α,β-unsaturated carbonyls from readily available building blocks without the generation of any stoichiometric byproducts in a redox-neutral, atom-economical process. This should be possible by employing a cycle which uses a Ti/Zr=O catalyst to couple a carbonyl with an alkyne (Figure 9); through, (i) [2+2]-cycloaddition between the [M]=O and an alkyne, (ii) insertion of a carbonyl into the [M]–C bond of the oxametallacyclobutene, and, finally, (iii) [4+2]-retrocycloaddition, releasing the α,β-unsaturated carbonyl product and regenerating the [M]=O catalyst. As the starting materials are already at the requisite oxidation states, the reaction will not require either a stoichiometric oxidant or reductant.
Figure 1. Proposed alkyne-aldehyde coupling reaction.
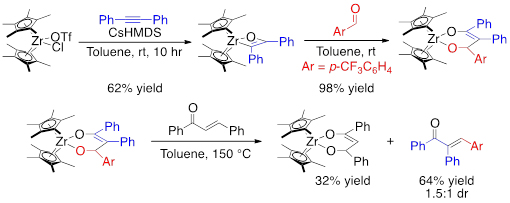
We have successfully demonstrated each step of the proposed catalytic cycle with Cp*2Zr=O (Cp* = pentamethylcyclopentadiene) complexes (Figure 10). The [2+2] cycloaddition is rapid at room temperature as is the insertion into aldehydes. The [4+2]-retrocycloaddition, however, requires 150 °C and is thermodynamically up-hill and reversible. Chalcone, an inexpensive natural product, can be added to trap the Zr=O and afford good to excellent yields of the α,β-unsaturated ketone.[1]The reaction is first order in dioxozirconacyclohexene and zero order in chalcone, indicating that the retrocycloaddition occurs to afford the Zr=O which then undergoes a rapid [4+2]-cycloaddtion with the less hindered α,β-unsaturated ketone selectively.
Figure 2. Zr=O medidated alkyne-aldehyde coupling.
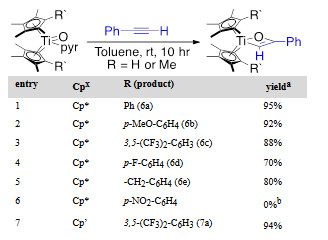
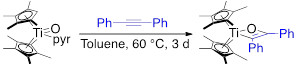
We have also sought to develop the analogous Cp*2Ti and Cp`2Ti (Cp` = tetramethylcyclopentadiene) mediated alkyne aldehyde coupling reaction. The both Ti=O complexes undergo rapid [2+2]-cycloaddition with terminal alkynes, though elevated temperatures are required for internal alkynes (Table 1 and Figure 3).
Table 1. Scope of terminal alkynes in [2+2] reaction.
Reaction condition: 1.0 equiv. titanocene–oxo, 1.2 equiv. alkyne; a Isolated yields; bStarting materials were consumed resulting in an intractable mixture.
Figure 3. Scheme 4. [2+2]-Cycloaddition reaction with internal alkynes
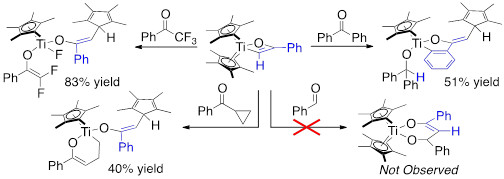
Unfortunately, under a variety of conditions the carbonyl insertion does not occur, rather a Lewis base promoted reductive elimination of the Cp*–C bond is observed; the resulting Cp*2Ti(II) complex undergoes rapid C–H, C–C, or C–X (X = F or Cl) activation (Figure 11).[2] Interestingly, the less hindered Cp`2Ti(II) complex does not react under a variety of reaction conditions, as only unreacted starting material is observed.
Figure 4. Examples of C–H, C–C, and C–F activation by Ti(II).
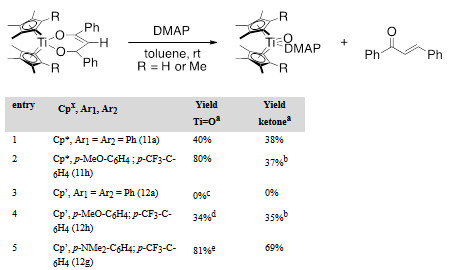
We proposed that these complexes could be prepared by [4+2]-cycloaddition reaction of oxo complexes with α,β-unsaturated carbonyls as similar reactivity has been reported with titanocene–sulfido complex Cp*2Ti=S(py). In the preparation of complexes dioxatitanacyclohexenes, it was noticed that with electron donating groups on both of the phenyl rings, the product was not stable and decomposed to the enone and metal–oxo cluster. This cluster was the decomposition product of [Cp*2Ti=O], generated from retro-[4+2] cycloaddition. It was hypothesized that other reagents which react irreversibly with titanium–oxo should also promote the generation of α,β-unsaturated ketones from dioxatitanacyclohexenes and afford stable Cp*2Ti=O(ligand) complexes. One class of such reagents is strong Lewis bases like 4-dimethylaminopyridine (DMAP), which can stabilize the monomeric titanocene–oxo species. Reactions of DMAP with dioxatitanacyclohexenes generated the corresponding enone and the DMAP-trapped titanium–oxo complexes in moderate to excellent yields (Table 2).
Table 2. Retro-[4+2]-cycloaddition of the dioxatitanacyclohexene.
Reaction condition: 1 equivalent metallacycle (0.02M in C6D6), 20 equivalents DMAP. a NMR yield determined by comparison of the product to an internal standard in 1H NMR spectra after 19 hours. b Lower yield of oxo compared to enone was attributed to low solubility of oxo product, as it precipitated out of solution. cNo reactions at 80 ˚C and longer reaction time. d Reaction was run at 60 ˚C. eYield determined after 5 hours.
Our stoichiometric studies have demonstrated that each step of the proposed catalytic cycle is possible with zirconium. Unfortunately, it also shown that the reaction is thermodynamically unflavored with Zr-complex and that one of the key-steps, the carbonyl insertion, does not occur with the Ti-complex.
Summary and Outlook: The overarching goals of the research in the Hull group are to develop practical methodologies that allow for the rapid synthesis of important functionalities. Our stoichiometric studies have demonstrated that each step of the proposed catalytic cycle is possible with zirconium. Unfortunately, it also shown that the reaction is thermodynamically unflavored with Zr-complex and that one of the key-steps, the carbonyl insertion, does not occur with the Ti-complex. Overall, we have made significant progress on investigating the Zr=O and Ti=O mediated alkyne aldehyde coupling reaction, demonstrated that the reaction is feasible stoichiometrically though would require additional efforts in ligand design to develop a catalytic protocol.
[1] Kortman, G. D.; Orr, M. J.; Hull, K. L. “Synthesis and Reactivity of Dioxazirconacyclohexenes: Development of a Zirconium–Oxo-Mediated Alkyne-Aldehyde Coupling Reaction,” Organometallics 2015, 34, 1013.
[2] Nguyen, T. T.; Bertke, J. A.; Gray, D. L.; Hull, K. L. “Facile C–H, C–F, C–Cl, and C–C Activation by Oxatitana-cyclobutene Complexes,” Organometallics 2015, 34, 4190.