Reports: UNI153776-UNI1: Development of a Palladium-Catalyzed Oxidative Aryl Transfer Reaction
William Brenzovich, PhD, Roanoke College
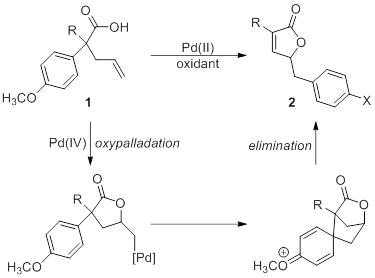
The major area of research in our lab has been in the development and exploration of reactions catalyzed by metals in a high oxidation state. Our lab’s specific aim is the study of a novel reaction catalyzed by palladium(IV) that involves the transfer of an aryl group. This reaction is unprecedented under palladium-catalysis and provides efficient access to an interesting structural motif, while offering insight into the reactivity of high-oxidation state palladium. We exploring the scope and mechanism of this reaction through careful examination of the steric and electronic requirements of the system. The information garnered from this project will be used to inform the development of additional new and novel palladium-catalyzed transformations.
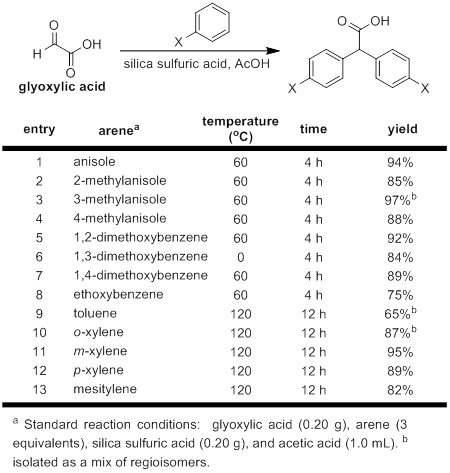
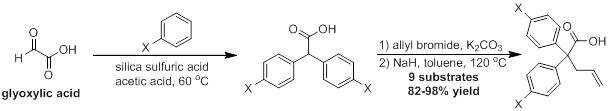
Research often moves in unexpected directions, and we were quickly forced to develop a new reaction in an effort to synthesize the required substrates for the reaction of interest. There are numerous syntheses of diarylacetic acids published in the literature; however, in our hands these methods proved either low-yielding or produced highly impure products. We therefore developed an alternative and more effective synthesis of these highly useful molecules through the use of a heterogenous catatlyst, silica sulfuric acid. This new protocol proved to be both high yielding and ammenable to a variety of electron-rich arene substrates. Even sterically encumbered arenes, such as mesitylene, provide good yields of the desired diarylacetic acid, though higher temperature and longer reaction times are required. This reusable catalyst system has a variety of different potential applications that will be subject of future work within our laboratory.
Following the synthesis of the requisite diarylacetic acids, instillation of the final carbon chain can be accomplished by the direct allylation of the acetic acid dianion; however, the sequence was unsuccessful for more sterically hindered substrates. A two-step esterification and Claisen rearrangement sequence was subsequently developed, allowing for the successful synthesis of ten substrates for testing under palladium catalysis.
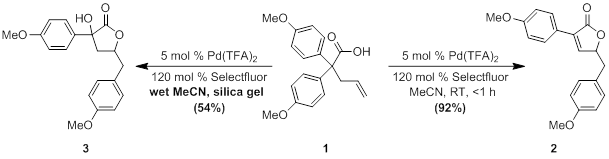
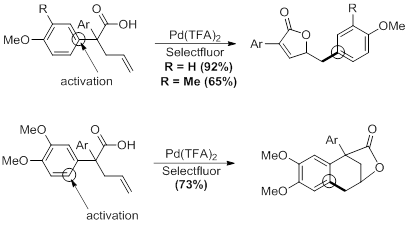
Optimized conditions, shown in Scheme X, for the palladium reaction were established through careful examination of various parameters. The reaction was found to be extraordinarily rapid, with complete consumption of starting materials occurring in under five minutes at room temperature. Selectfluor was the only oxidant studied that produced the product.
During the optimization studies, an additional interesting by-product (3) was observed, resulting from the addition of water rather than elimination. Preliminary experiments have confirmed that it does not result from the decomposition of the product. The complete stereochemical nature of the reaction has yet to be determined, and we are currently attempting to crystalize this compound for X-ray analysis. The formation of the byproduct has been minimized by the use of dry solvents, though the reaction can be shifted to form only the alcohol product through the addition of wet silica gel to the reaction mixture. This new product offers an additional avenue in the exploration of this palladium reaction.
The course of the reaction is highly dependent on the substitution pattern of the arene. The expected unsaturated lactone product is formed in reasonable yields as long as activating groups are present in the ortho- and para-positions of the aromatic ring. However, when a highly activating group is placed in the meta-position, a change in reaction pathway occurs, and an alternate tricyclic product is formed. Currently, we are looking into similar substrates with this dual activation to determine at what point the selectivity in the reaction changes. In addition, we are currently investigating the synthesis of substrates with substitution only in the 3-position to further confirm our observations.
Over the past year, our group has successfully developed a facile and rapid synthesis for the required substrates to explore the palladium reaction of interest, involving the development of a heterogeneous reaction catalyzed by silica sulfuric acid. We have optimized the general conditions for the palladium reaction, and have begun completion of our intended study of the reaction scope relative to the substitution of the aromatic ring. In the process, we have found that the reaction is consistent with a nucleophilic mechanism, requiring an electron-donating group in either the ortho- or para- position. The addition of an activating group in the meta-position of the aromatic ring results in the formation of an alternative tricyclic product. In the coming year, we will begin to explore more specific mechanistic studies to shed further light on the nature of the reaction. While many of our planned research goals have been met, our studies have raised even more questions about the nature of this reaction, providing ample areas for further investigations.
As we are located at a small liberal arts institution, all laboratory was conducted by undergraduate students. Generous ACS-PRF support has enabled three different students to have a fully funded summer research experience, while providing research supplies for an additional four. Students who have worked on this research project intend to enroll in graduate studies in the chemical sciences, seeking both terminal Masters and Ph.D. degrees. Students have gained valuable laboratory experience, learning the technical skills as well as gaining a deeper understanding of chemistry. This research has culminated in numerous poster presentations at local and regional conferences in addition to two undergraduate posters at the Spring 2015 National American Chemical Society conference in Denver. In addition, two manuscripts are in preparation for the dissemination of our results.