Reports: UR152798-UR1: Synthesis of Azaannulenes Related to the Porphyrins
Timothy D. Lash, Illinois State University
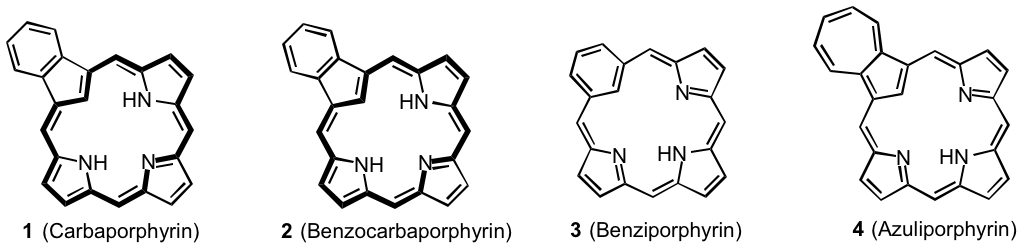
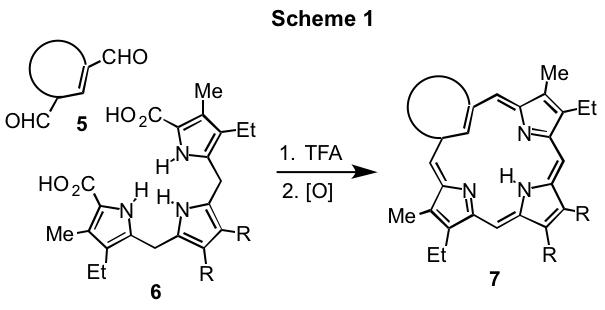
Carbaporphyrinoids such as 1-4 exhibit diverse characteristics and may exist as strongly aromatic, nonaromatic or even antiaromatic species.1,2 These systems commonly form organometallic derivatives under mild conditions and exhibit unusual reactivity.1,2 In addition, derivatives of benzocarbaporphyrins 2 have been shown to be effective agents in the treatment of leishmaniasis.3 Carbaporphyrinoids can be synthesized by a "3 + 1" variant on the MacDonald reaction where an aromatic dialdehyde 5 is condensed with a tripyrrane 6 in the presence of TFA, and following an oxidation step carbaporphyrinoids 7 are often isolated in good yields (Scheme 1). Although this strategy has been very successful, multiple steps are commonly required to prepare the tripyrrane intermediates. In addition, an oxidation step is necessitated that is not always straightforward.4 Therefore, not only would it be of value to have a more direct route to tripyrrane-like intermediates but it would also be beneficial if a methodology could be developed that avoided the requirement for an oxidation step.
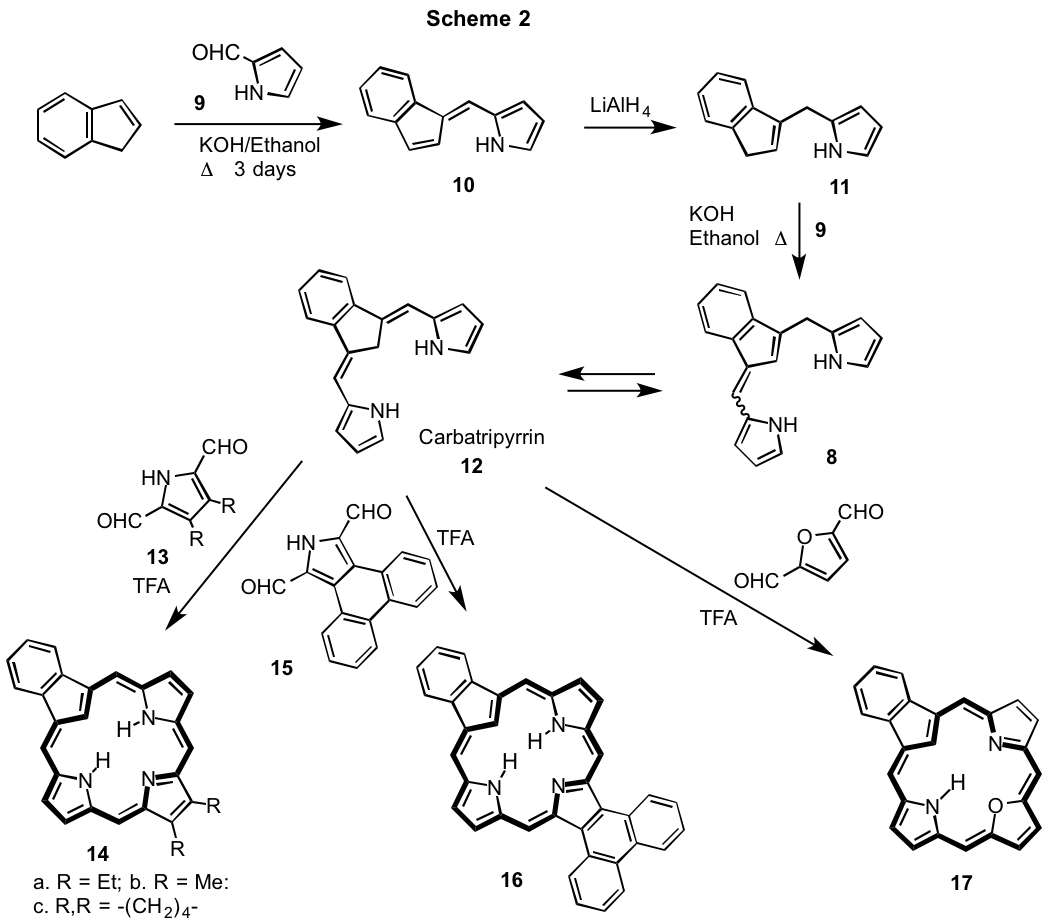
We speculated that if a tripyrrene-like intermediate similar to 8 could be isolated in the Z-configuration, this system would be geometrically preorganized to facilitate macrocycle formation and importantly the oxidation step would no longer be needed.5 Reaction of pyrrole-2-carbaldehyde (9) with technical grade indene in refluxing KOH-ethanol gave fulvene product 10 in 92% yield. Reduction of 10 with LiAlH4 in refluxing THF then gave a dihydro-derivative that primarily consisted of structure 11 in 74% yield. This crude product was reacted with 9 in refluxing 1% KOH-ethanol gave tripyrrenes 8 as a mixture of E- and Z-isomers. However, at higher concentrations a carbatripyrrin 12 precipitated out from the reaction mixture in 75% yield.5 As carbaptripyrrin 12 is geometrically compatable with porphyrinoid synthesis, it was reacted with pyrrole dialdehyde 13a in dichloromethane with catalytic TFA (Scheme 2) and following work up, purification by column chromatrography and recrystallization from chloroform-methanol, carbaporphyrin 14a was isolated in 51% yield. Reaction of 12 with pyrrole dialdehydes 13b and 13c similarly gave the related carbaporphyrins 14b and 14c in 29% and 20% yield, respectively. Carbatripyrrin 12 was also condensed with phenanthropyrrole dialdehyde 15 to give phenanthrocarbaporphyrin 16 in 22% yield (Scheme 2). Furthermore, condensation of 12 with 2,5-furandicarbaldehyde gave oxacarbaporphyrin 17 in 43% yield (Scheme 2). Given that carbatripyrrin can be prepared from commercially available materials in 50% yield, this strategy provides a superior route to carbaporphyrinoid systems.5
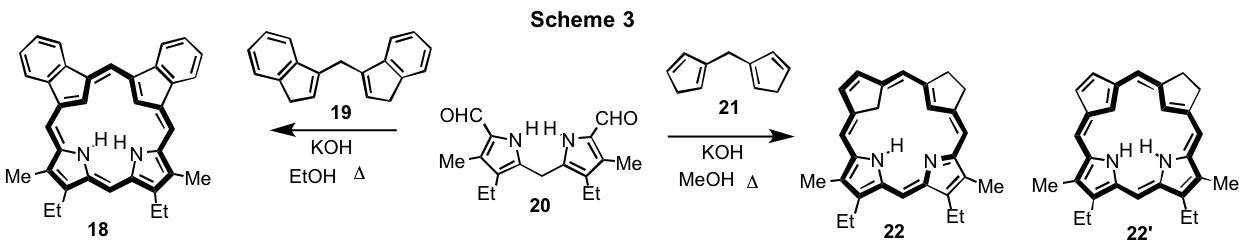
As carbaporphyrins have such interesting properties, porphyrin analogues with two carbocyclic subunits have also been investigated.6,7 Recently, a base-catalyzed MacDonald reaction was used to prepare adj-dicarbaporphyrin 18 by reacting diindenylmethane 19 with dipyrrylmethane dialdehyde 20 in the presence of KOH in refluxing ethanol (Scheme 3).7 This remarkable system reacted with palladium(II) acetate to give a unique tripalladium sandwich complex encapsulating a Pd(IV) ion.7 In order to further investigate dicarbaporphyrins of this type, dicyclopentadienylmethane 21 was reacted with 20 and KOH in refluxing methanol (Scheme 3).8 The best results were obtained when the reaction was carried out for 4 days using excess 21 and a dicarbachlorin product 22 was isolated in 5-7% yield. The UV-vis spectrum for this product was very similar to porphyrins or carbaporphyrins such as 21 in that is showed a strong Soret band at 398 nm and a series of Q bands at 501, 533, 619 and 683 nm. The proton NMR spectrum of 22 in CDCl3 indicated that an asymmetrical porphyrinoid product had been generated with upfield resonances at -6.68 (2H) and -4.03 ppm (1H) for the internal CH2 and CH units, respectively. These results clearly showed that the compound corresponded to 22 rather than tautomer 22’ with two internal NHs, and confirmed that the first example of a carbaporphyrinoid system with an internal methylene unit had been generated.8 The structures of 18 and 22 were confirmed by X-ray crystallography.8 The dicarbachlorin system represents a new, albeit long anticipated, structural variation for porphyrin analogues and opens up new possibilities for carbaporphyrin research.
References
1. Lash, T. D. Recent advances in the synthesis and chemistry of carbaporphyrins and related porphyrinoid systems, Eur. J. Org. Chem. 2007, 5461-5481.
2. Lash, T. D. Benziporphyrins, a unique platform for exploring the aromatic characteristics of porphyrinoid systems, Org. Biomol. Chem. 2015, 13, 7846-7878.
3. Taylor, V. M.; Cede–o, D. L.; Mu–oz, D. L.; Jones, M. A.; Lash, T. D.; Young, A. M.; Constantino, M. H.; Esposito, N.; VŽlez, I. D.; Robledo, S. M. Antimicrob. Agents Chemother. 2011, 55, 4755-4764.
4. Li, D.; Lash, T. D. J. Org. Chem. 2014, 79, 7112-7121.
5. Stateman, L. M.; Lash, T. D. Syntheses of carbaporphyrinoid systems using a carbatripyrrin methodology, Org. Lett. 2015, 17, 4530-4533.
6. a. Lash, T. D.; Romanic, J. L.; Hayes, M. J.; Spence, J. D. Towards Hydrocarbon Analogues of the Porphyrins: Synthesis and Spectroscopic Characterization of the First Dicarbaporphyrin, Chem. Commun. 1999, 819-820. b. Lash, T. D.; Colby, D. A.; Idate, A. S.; Davis, R. N., Fulvene dialdehyde strategy for adj-dicarba-porphyrinoid synthesis: preparation of a 22-carbaazuliporphyrin, J. Am. Chem. Soc. 2007, 129, 13801-13802. c. Lash, T. D.; Lammer, A. D.; Idate, A. S.; Colby, D. A.; White, K. Preparation of azulene-derived fulvenedialdehydes and their application to the synthesis of stable adj-dicarbaporphyrinoids, J. Org. Chem. 2012, 77, 2368-2381. d. Lash, T. D.; Lammer, A. D.; Ferrence, G. M. Two-step synthesis of stable dioxadicarbaporphyrins from bis(3-indenyl)methane, Angew. Chem. Int. Ed. 2012, 51, 10871-10875.
7. AbuSalim, D. I.; Ferrence, G. M.; Lash, T. D. “Synthesis of an adj-dicarbaporphyrin and the formation of an unprecedented tripalladium sandwich complex”, J. Am. Chem. Soc. 2014, 136 (18), 6763-6772.
8. Lash, T. D.; AbuSalim, D. I.; Ferrence, G. M. “adj-Dicarbachlorin, the first example of a free base carbaporphyrinoid system with an internal methylene unit”, Chem. Commun. 2015, in press. DOI: 10.1039/c5cc06890h