Reports: ND453795-ND4: Design, Synthesis and Properties of Functional Dyes Based on the Peri-Napthindigo Template
Robin G. Hicks, University of Victoria
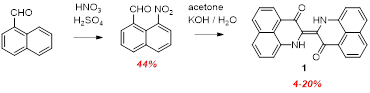
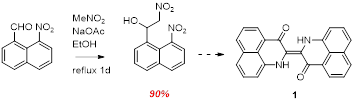
The primary objectives and achievements to date have focused on discovering and optimizing efficient synthetic routes to “peri-naphthindigo” (PNI) and its derivatives. We discovered that Baeyer-Drewson type reaction of 8-nitro-1-naphthaldehyde with acetone under basic conditions produced the parent PNI 1 in low yield (Scheme 1). As anticipated, this molecule is an intensely absorbing green chromophore (absorption maximum 647 nm, extinction coefficient ~25,000). However, and not totally unexpectedly, this flat species is quite insoluble in all common solvents.
Scheme 1
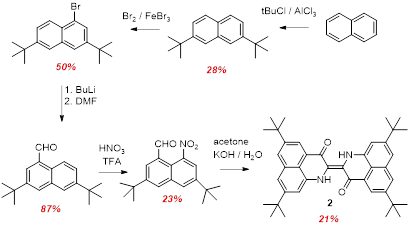
We therefore decided to attempt to prepare a more soluble analogue through peripheral substitution. The tetrakis-tert-butyl derivative 2 could be prepared analogously, see Scheme 2. Once again the final step (condensation / coupling reation of the nitro aldehyde) was relartively low yielding. The product 2 was indeed more soluble than the parent compound 1; the electronic spectra of 1 and 2 are virtually identical.
Scheme 2
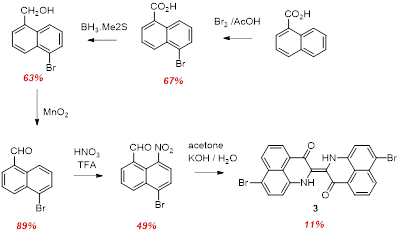
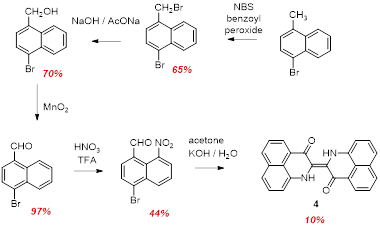
We have also synthesized two brominated derivatives which we targeted for post-synthetic coupling chemistry to append pi conjugated substituents. As was the case for the tert-butyl compound, multi-step (ut straightforward) precursor chemistry afforded two isomers of the bromo- substituted nitro aldehydes. These could be converted into the corresponding dibromo PNI compounds 3 and 4 in low yields.
Scheme 3
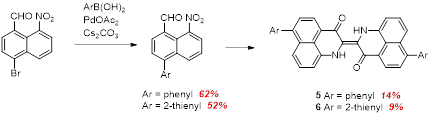
As mentioned above, the two dibromo PNI compounds were desired to subsequently extend the pi conjugated system outward via cross-coupling chemistry. However, the multistep nature and low yield of the final PNI synthesis step led us to consider an alternative way to achieve this. We have found that the bromo nitro aldehydes could be coupled with aryl boronic acids to give the corresponding aryl derivatives, which we could then convert to PNI derivatives (Scheme 5). Gratifyingly the Suzuki coupling reaction does not appear to have any deleterious effect on the ntro aldehyde functionality. Preliminary studies indicate the thiophene compound in particular has a significant red-shift in its electronic absorption spectrum.
Scheme 5
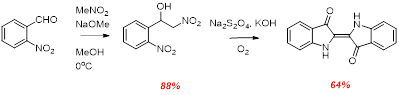
The Bayer-Drewson reaction to make PNI (this is the same reaction used in the original synthesis of the famous pigment indigo, using ortho nitrobenzaldehyde in place of our nitro naphthaldehyde) clearly has become a serious bottleneck to further progress. We decided to invest in exploration of a superior route to these compounds.
We recently unearthed a modification of the original Baeyer-Drewson indigo synthesis. The first step of this reaction is a Henry type reaction involving nitromethane conjugate base in place of the acetone anion as nucleophile. The Henry reaction product is normally isolated (unlike the aldol reaction product of the Bayer-Drewson reaction) and then trated with dithionite under basic conditions, followed by aerobic oxidative coupling to give indigo. Notably this procedure gives indigo in a much higher yields compared to the B-D reation (yields typically are ~10%).
Scheme 6
Emboldened by this discovery we have most recently turned to applying these synthetic protocols to the nitro naphthaldehyde species in the hopes of improving PNI synthesis. After some initial challenges, modified reaction conditions (compared to those used for nitro benzaldehyde) gave the Henry addition product in excellent yield (Scheme 7). Experiments are underway to optimize the PNI synthesis – early indications are very promising.
Scheme 7