Reports: ND352709-ND3: New Homoleptic Rare-Earth Metal Complexes for Catalytic Hydrophosphination
Joseph A. R. Schmidt, PhD, University of Toledo
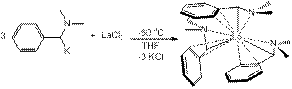
Joseph A. R. Schmidt, PhD, University of Toledo
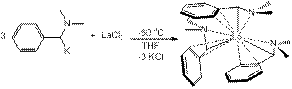
Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society