Reports: UR354579-UR3: BODIPY-Pd Complexes for Photocatalytic C-C Coupling Reactions
Hongshan He, PhD, Eastern Illinois University
The objective of this project is to test the hypotheses that BODIPY (BODIPY= 4,4'-difluoro-4-bora-3a,4a-diaza-s-indacene) chromophore can activate Pd(II) through an intramolecular electron transfer process for catalytic Sonogashira C-C coupling reaction under visible light illumination. We plan to prepare new BODIPY-modified 1,10-phenanthroline ligands and their Pd(II) complexes to test this hypothesis. The absorption, fluorescence, and electrochemical properties will be determined to reveal the energy transfer process. The theoretic calculations will be performed to provide insights on energetics of intermediates. Detailed photocatalytic properties of the Sonogashira C-C coupling reactions will be investigated to reveal the reaction mechanism.
In Year 1, one graduate student and one undergraduate student participated in the project. Both students obtained significant training on manipulation of air-sensitive materials sing Schlenk lines and glovebox. They also received complete training on column chromatography as well as skills on growing single crystals. Several major scientific accomplishments are summarized in the following. Some of these results have been presented at the ACS 2015 Joint Great Lakes/Central Regional Meeting.
1) Two BODIPY ligands and Pd complexes have been synthesized and characterized.
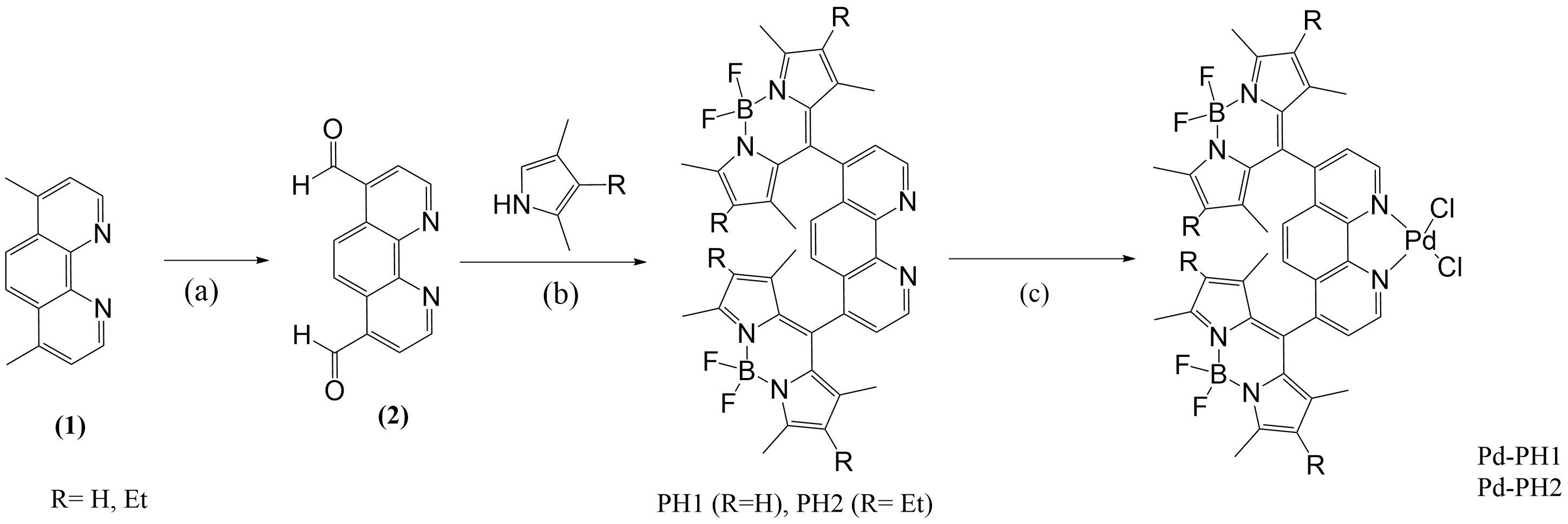
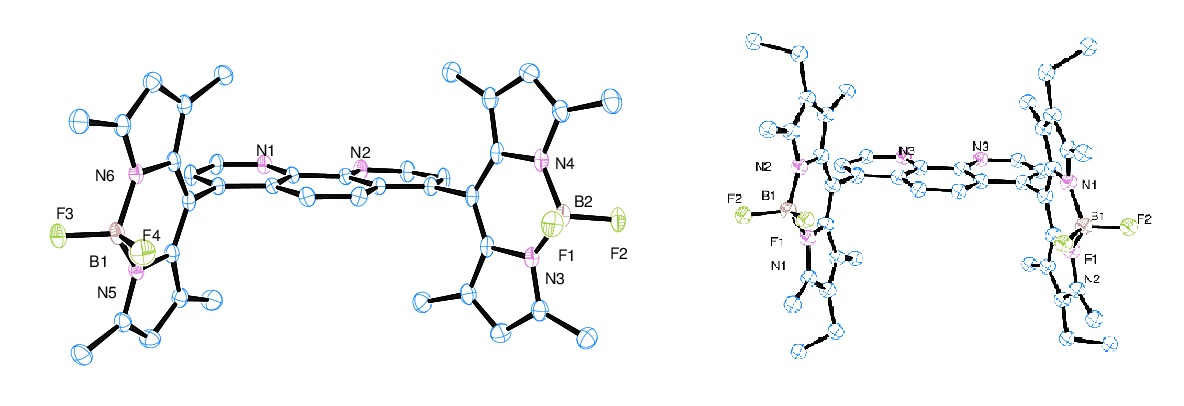
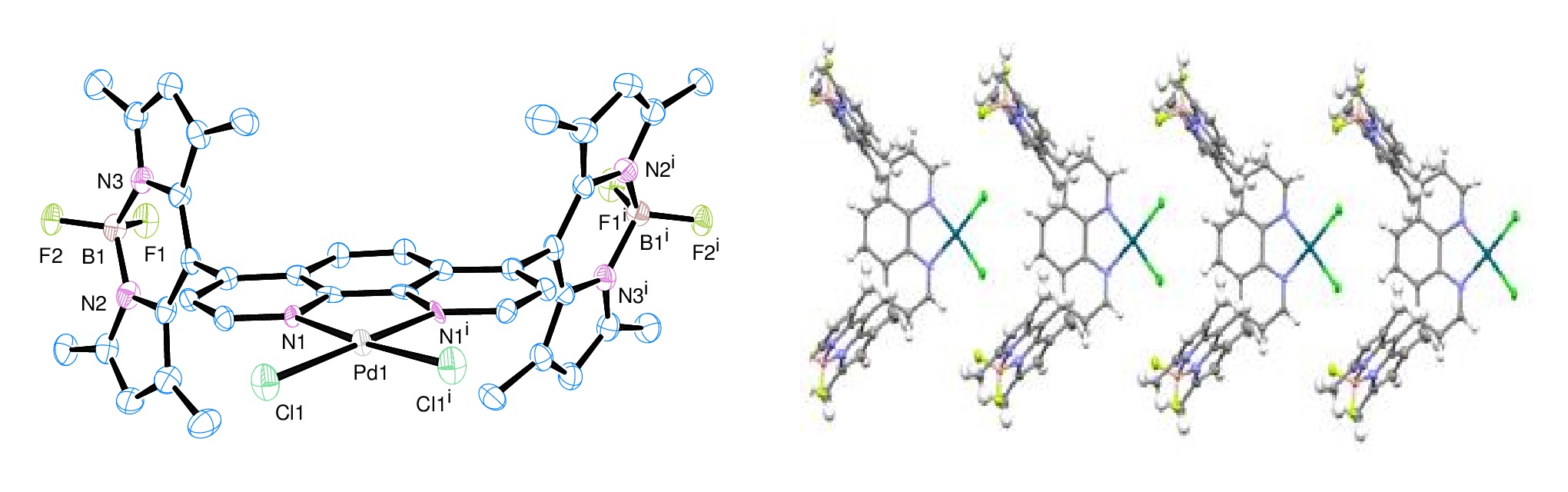
Figure 1 shows the synthetic procedure of two BODIPY dyes, PH1 and PH2 and their Pd (II) complexes. The conversion of precursor (1) to dialdehyde (2) was straight forward with yields ~ 60%. Formation of PH ligands was carried out in “one-pot” reaction mode with yields ~ 30% after three-consecutive column chromatographic purification; therefore significant efforts were put on the production of these ligands. The structure of two ligands was characterized by 1H NMR spectroscopy and single-crystal X-ray diffraction analysis. Figure 2 shows the ORTEP diagrams of two ligands, PH1 and PH2. Two ligands exhibit very similar structural characteristics. In each ligand, two BODIPY units are aligned almost vertically to the 1,10-phenanthroline unit. In PH2, four ethyl groups are pointed outwards. The Pd (II) complex was prepared by direct interaction of Pd(PhCN)2Cl2 with ligand in CH2Cl2 at room temperature for 24h. The yield was about ~ 70%. The single crystal structure was shown in Figure 3. As expected, the Pd (II) is four-coordinate in a square planar geometry with two N atoms from the 1,10-phenanthroline unit and two Cl- anions.
Figure 1. Synthesis of BODIPY ligands and their Pd (II) complexes. Reaction conditions: (a) SeO2, 1,4-dioxane, reflux; (b) (i) TFA, CH2Cl2, r.t.; (ii) DDQ, r.t. ; (iii) Et3N; (4) BF3▪OEt2; (c) Pd(PhCN)2Cl2, r.t
Figure 2. ORTEP diagrams of PH1 (left) and PH2 (right) with 50% thermal ellipsoid probability. Hydrogen atoms were omitted for clarity
Figure 3. Left: ORTEP diagrams of Pd-PH1 with 50% thermal ellipsoid probability. Hydrogen atoms were omitted for clarity; Right: packing of Pd-PH1 along axis b.
2) Absorption and fluorescence of ligands and Pd (II) complexes.
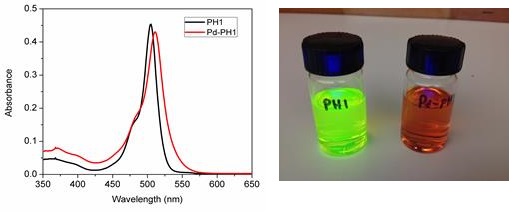
The ligands PH1 and PH2 exhibited very strong absorption around 500 nm, which is very typical for BODIPY dyes. After forming complexes with Pd (II), the absorption peak of PH1 red-shifted from 512 nm to 520 nm in CH2Cl2 at room temperature. A similar change was also observed for PH2. The ligand showed very strong fluorescence under UV illumination; the fluorescence was almost completely quenched in the Pd (II) complex as shown on Figure 4. This indicated clearly that Pd (II) induced or altered the fluorescence decay pathways. It is unclear at this moment if this is caused by electron transfer from BODIPY to Pd (II) center. We will study this effect in details in Year 2 and Year 3.
Figure 4. Left: Absorption spectra of PH1 and Pd-PH1 in acetonitrile; Right: Images of solutions of PH1 and Pd-PH1 in CH2Cl2 under UV excitation
3) Theoretical calculations for two ligands were carried out at XSEDE facilities.
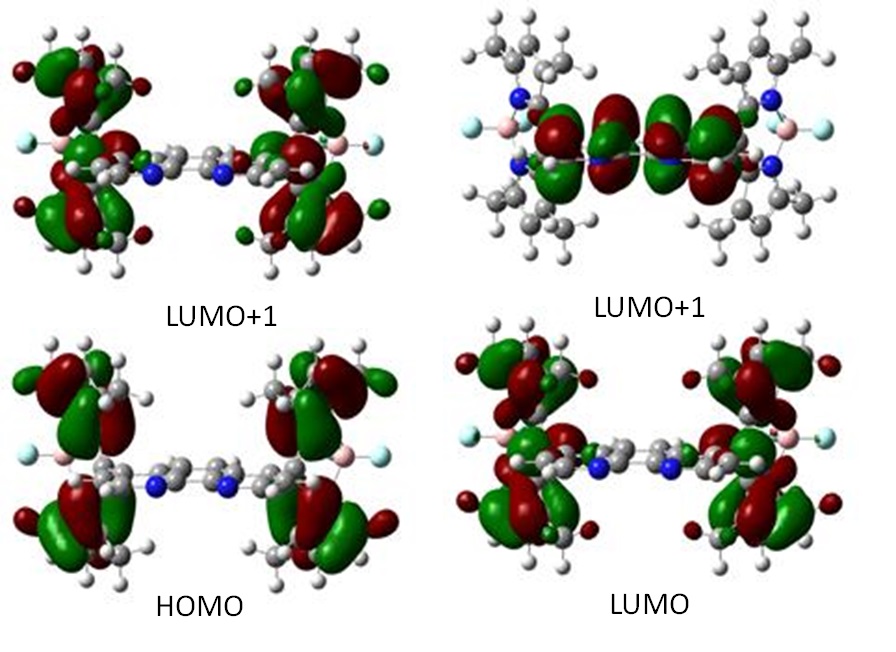
This was proposed as the task for the Year 2; however we started this task early while we are making ligands and complexes. The calculations were performed on XSEDE cluster (Blacklight @ PSC and Trestles@ SDSC) through the grant TG-CHE130116 that was awarded to PI He. The calculations were performed on density functional theory level using B3LYP/6-31g(d) in acetonitrile. The optimized structures were very similar to the single-crystal structures. The electron densities of HOMO and LUMO were both centered on BODIPY core with a slight shift toward 1,10-phenanthroline as shown in Figure 5. However, the electron density of LUMO+2 was completely on 1,10-phenanthroline indicating that BODIPY unit will be most likely the electron provider for the reduction of Pd (II).
Figure 5. Electron density distribution profiles of HOMO and LUMO.