Reports: DNI454435-DNI4: Absolute Stereocontrol of Prochiral Substrates with Chiral Excited State Proton Transfer Dyes
Kenneth Hanson, PhD, Florida State University
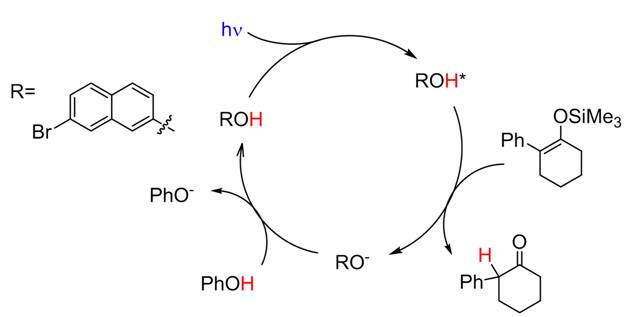
The reaction goes to near completion (96% yield) under a nitrogen
atmosphere with only 1 mol % of Br-NpOH as the photocatalyst. The 96 % product yield indicates that we can
achieve at least 96 turnovers per photocatalyst. The
reaction does not occur in the absence of light even at elevated temperature
and thus the reaction progression can readily be controlled by light
modulation. Irradiating a solution of 1-phenyl-2-(trimethylsiloxy)cyclohexene, naphthol (50 mol %),
SENS (2.5 mol %), and sacrificial acid (AH in excess) with 445 nm light gave
product in 74% yield. This reaction yield corresponds to ~30 and ~1.5 turnovers
for the sensitizer and ESPT dye, respectively. The sensitized photocatalytic reaction has several advantages over the unsensitized cycle. The first is that unsubstituted
naphthol, which is much more chemically robust and
can be found in coal tar, can be used as the ESPT catalyst. The second
advantage is that due to the small singlet-triplet gap of SENS can shift the
excitation to the visible region and still have sufficient excited state energy
to drive the reaction. The above results are first example of an organic
transformation via direct and sensitized excited state proton transfer
catalysts. These results open the door to an entirely new class of photocatalytic reactions that harness the acidity of
excited state proton transfer dyes. A manuscript containing these results has been submitted and is
currently under review for publication in the Journal of the American
Chemical Society. Anjan Das, who is the first
author on the manuscript, presented these results at the Gordon Research
Conference on Photochemistry and his poster received considerable attention
from both synthetic organic and photophysical
chemists. Additionally, over the past year, Anjan, who
came into this research as a synthetic organic chemist, has gained considerable
knowledge in molecular photophysics and will have a
diverse and relatively unique set of skills as he enters the job market search next
year. This is particularly important given the increasing interest in photocatalysis as a green alternative to traditional synthetic
organic chemistry. Upon successfully demonstrating the feasibility of ESPT catalysts, we
have shifted our focus to performing the protonation
reaction enantioselectively. As outlined in our
proposal our first attempts were primarily focused on BINOL derivatives but
found that despite several different types of substitution, the dye to planarized and decomposed upon irradiation. As an alternative
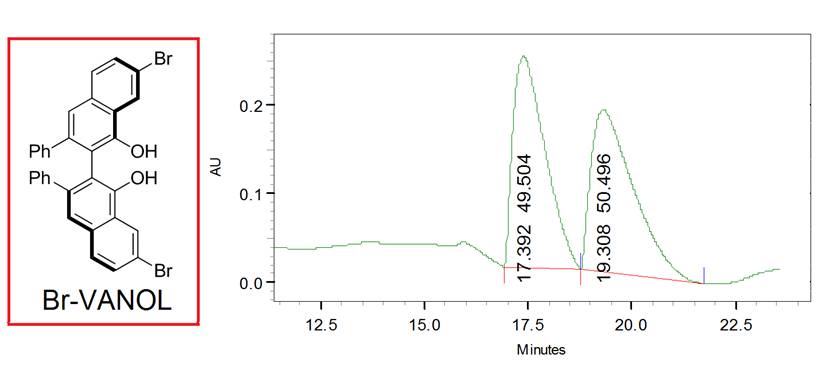
to BINOL, Br-VANOL ((R)-4,4'-Dibromo-3,3′-Diphenyl-2,2′-bi-1-naphthol)
is a molecule that is chiral has alcohol groups, the bromine
atoms necessary to access the triplet state and significant steric
bulk to prevent planarization. Using Br-VANOL in the place of Br-NpOH in the catalytic cycle above resulted in a 36% yield
with a 40 % enantiomeric excess of (S)-2-phenylcyclohexanone
as determined by HPLC shown below. As far as we know this is the first demonstration
of enantiopreferential protonation
of a prochiral substrates via chiral
ESPT catalysis.