Reports: UR451677-UR4: Electron Driven Formation of Aromatic and Nonaromatic Substituted Isocyanurates and Isocyanuratocyclophanes from Isocyanates
Steven J. Peters, PhD, Illinois State University
One electron reduction of isocyanates. The
remarkable utility of isocyanates (R of the extensive chemical research already performed
using isocyanates and isocyanurates, there is very little known about the one
electron reduction of either of these systems. We have been particularly
interested in generating isocyanate anion radicals for spectroscopic
investigation to explore the similarities, if any, with the structure and the
electron-spin distribution found in ketyls (e.g., RNCO• My students and I continued to explore the one electron
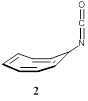
reduction of isocyanates with emphasis on systems that have Cyclooctatetraenyl Isocyanate (COTNCO).
To further our understanding of the stability and the reactivity of
isocyanate anion radicals, my group and I were motivated to investigate the one
electron reduction of COTNCO (2). The synthesis of 2 had not yet been reported. Therefore, a former graduate
student (Joseph Klen) and I developed a synthetic route to this new isocyanate
starting with cyclooctatetraene. We were interested in this system because of
the high electron affinity of the [8]annulenyl moiety in 2. We
anticipated that this would result in much of the electron density residing
within the [8]annulenyl ring upon addition of an electron. Since there is a
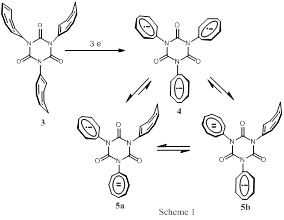
fundamental quantum mechanical difference between COT (4n The electron reduction of COTNCO was found to produce the
corresponding isocyanurate species (3) (see Scheme 1). Unlike the
previously studied tri-phenyl isocyanurate anion radical, the unpaired electron(s)
was found to localize within a COT moiety attached to the isocyanurate ring.
Further exposure to metal results in the formation of an equilibrium mixture of
trianion triradical (4) and trianion radical (5a,b), see
Scheme 1. The formation of 5a,b is a consequence of
intramolecular disproportionation. Interestingly, the COTNCO anion radical was
never observed in these studies. We believe this is a consequence of the favorable
disproportionation of the anion radical to the COTNCO dianion. The additional
charge (resulting
from the added second electron) would make the COT
dianion a powerful nucleophile. We proposed that this dianion drives the
cyclotrimerization to form the isocyanurate species (3). Remarkably, we
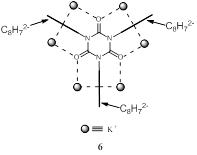
found that exhaustive reduction of the tris-COT isocyanurate in THF generates
the first-ever observed hexa-anion of an isocyanurate. NMR analysis revealed
that each of the three planar D8h COT dianion moieties
form tight ion pairs with two counter K+ ions (6).
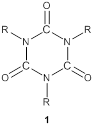
1-Naphthyl Isocyanate (1-NapNCO). As
described above, my group has found that exposure of an alkyl or aryl
isocyanate solution to an alkali metal (either potassium or sodium) is an
effective and rapid method of cyclotrimerization leading to the generation of
the respective isocyanurate. This process of isocyanurate formation has been
proposed to proceed via the reactive isocyanate anion radical, formed from the
one-electron reduction of the isocyanate, which rapidly attacks two more
neutral isocyanates. In the case of aryl isocyanates, this protocol is
effective at converting phenyl isocyanate (PhNCO) and a number of para-substituted
PhNCO compounds to their respective isocyanurates. However, prior to the
studies with 1-NapNCO we had yet to determine the efficacy of converting
isocyanates with bulky substituents attached to their respective cyclotrimers.
The one electron reduction experiments on this compound were performed by my
former student Mark Kassabaum (MS, ‘12). We determined that exposure of a
solution containing 1-NapNCO did indeed result in the rapid cyclotrimerization
to the respective isocyanurate in very good yield. What was somewhat
surprising to us initially is that two isomeric forms of the tris-(1-napthyl)
isocyanurate are generated in the reaction. Upon closer inspection we found
that these isomers are diastereomers that can be classified further as atropisomers.
Atropisomers are two or more diasteroisomers generated as a result of hindered
rotation of the bulky substituents attached. In the case of tris-(1-naphthyl)
isocyanurate, hindered rotation of the three naphthyl rings produces two
diasteroisomers, which are easily distinguishable using NMR spectroscopic techniques.
Michael Nocella (BS ‘11) worked with me in the separation and purification of
the two different diasteroisomers and he successfully grew single crystals of
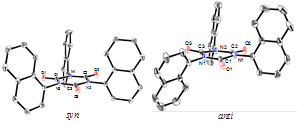
each for structural analysis via X-ray crystallography. Shown below are the
ORTEP diagrams of the two isomers (syn and anti), which are distinguishable
by the orientation of the naphthyl rings. Finally, in these studies we were
able to detect a small amount of 1-NapNCO anion radical present in solution.
As in our previous studies, we proposed that this anion radical initiates and
propagates the formation of the two atropisomers of the cyclotrimer.