Reports: UR151036-UR1: Applications of Iron(III) Compounds: Catalysts for Organic Synthesis
Ram S. Mohan, Illinois Wesleyan University
Iron(III) p-toluenesulfonate (tosylate) is an efficient catalyst for acetylation of alcohols, phenols, and aldehydes. The acetylation of 1º and 2º alcohols, diols, and phenols proceeded smoothly with 2.0 mol % of catalyst. However, the reaction worked well with only a few 3º alcohols. The methodology was also applicable to the synthesis of a few benzoate esters but required the use of 5.0 mol % catalyst. Aldehydes could also be converted into the corresponding 1,1-diesters (acylals) under the reaction conditions.
Iron(III) tosylate is an inexpensive, and easy to handle, commercially available catalyst.
2. Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via the Biginelli reaction
The synthesis of dihydropyrimidinones and dihydropyrimidine thiones has attracted interest due to their biological activities. A common method for their synthesis is the Biginelli reaction, which is a one-pot condensation of an aryl aldehyde, urea (or thiourea), and ethyl acetoacetate. The Biginelli reaction is typically catalyzed by a Brönsted or Lewis acid. However, many of these catalysts such as BF3.Et2O and AlCl3 are corrosive and/or toxic. Our continued interest in environmentally friendly organic synthesis prompted us to investigate the utility of iron(III) tosylate as a catalyst for the Biginelli reaction. The use of acetals in the Biginelli reaction is also reported. Iron(III) tosylate is an attractive catalyst for the Biginelli reaction because of its low cost, low toxicity, and ease of handling.
3. Bismuth compounds in organic synthesis. Bismuth bromide catalyzed one-pot synthesis of polyhydroquinoline derivatives.
We first investigated the iron(III) tosylate catalyzed one-pot synthesis of polyhydroquinolines via a multicomponent synthesis (The Hantzsch reaction). However, initial results were not as promising as they were for some other reactions carried out in the group using iron(III) tosylate. Due to our interest in polyhydroquinoline, we continued screening other catalysts and found that the best results were obtained with bismuth(III) bromide. All reactions were carried out at room temperature or 50° C when slow at room temperature. Product was obtained in good to excellent yields after recrystallization or simply washing with ethanol/water (1:1). The use of a non toxic bismuth salt, and a relatively green solvent, ethanol make this method particularly attractive from a green chemistry perspective. This work has now been published.
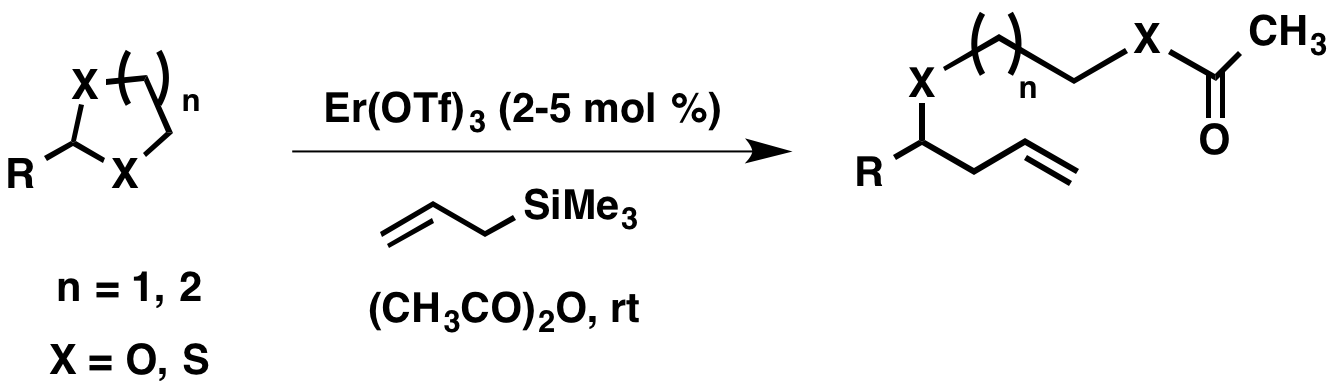
4. Erbium triflate catalyzed allylation of cyclic acetals
Although the allylation of simple acetals is well documented in the literature, fewer methods have been reported for the allylation of cyclic acetals. Many of the reported methods utilize reagents that are either corrosive (for example, titanium tetrachloride) or toxic (for example, organotin reagents). As part of our catalyst screen, we first tested the utility of iron(III) tosylate as a catalyst for these reactions. The allylation was successful only when the putative alkoxide was derivatized using an anhydride. While it showed promise, we found erbium triflate to be a superior catalyst for the allylation of a variety of cyclic acetals followed by
Scheme 1
Impact of PRF grant on PI and students
The impact of the generous funding from PRF on both the PI and students has been multifold and is summarized here:
(1) The grant has allowed me to mentor students during summers and this in turn has kept my research program moving. While we were able to accomplish the stated outcomes and demonstrate the utility of iron(III) salts as catalysts for organic synthesis, an unexpected outcome was the discovery that erbium slats are promising catalysts as well. This is now beginning to shape the new direction in which I hope to take my research. We are screening not only erbium salts, but a few other lanthanide salts as well for various organic reactions.
(2) The grant has allowed students to get a meaningful summer research experience. Due to the stipend provided by the grant, students were able to focus on research in the summer and work uninterrupted by course work (which happens during the academic year). Working on a project for 40 hours per week also gives them a more realistic feel for what graduate school will be like.
(3) The grant allowed me and four students to attend the National ACS meeting in Boston this Fall (2015). Students were very excited to attend the meeting, the various talks and poster sessions and interact with PhD chemists from both academia and industry. Two of the students presented their poster in the organic division and this boosted their confidence and also has inspired them to do more research. They have all returned determined to pursue graduate studies in organic chemistry. In my view, this is a major impact on them and was possible only because of the travel support provided in the PRF grant.