Reports: UNI754467-UNI7: Designing 'Smart' Polymer Self-Assembling Systems by Tuning Polymer Functionality and Architecture
Elizabeth M. Glogowski, PhD, University of Wisconsin - Eau Claire
Introduction and Goals
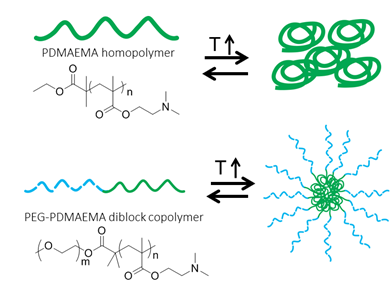
Smart polymers are polymers that change properties in response to an external stimulus such as temperature or pH. Poly((2-dimethylaminio)ethyl methacrylate) (PDMAEMA) is a smart polymer that is water-soluble at low temperature and low pH and water-insoluble at high temperature and high pH. The goal of this project is to determine the impact of both polymer chemical functionality and polymer architecture on smart properties of PDMAEMA. The cloud point, or the temperature at which PDMAEMA changes solubility and polymer aggregation is visibly occurring, is directly affected by the polymer structure. In addition to affecting stimuli-responsive behavior such as cloud point, changing smart polymer structure will also affect the structure of the aggregated polymers. While the impact of polymer structure on PDMAEMA homopolymer stimuli-responsive behavior has been studied extensively, reports on PDMAEMA diblock copolymers are more limited. A diblock copolymer of the smart PDMAEMA with the hydrophilic polyethylene glycol (PEG) will result in different aggregated structures above the cloud point compared to the PDMAEMA homopolymer, as illustrated in Figure 1. Changing the polymer structure also results in the ability to tune the cloud point over a wide range of temperatures, from 10°C to 85°C.
Figure 1. Reversible aggregation of PDMAEMA homopolymer (top) and PEG-PDMAEMA diblock copolymer (bottom) in response to a change in temperature.
Results Progress
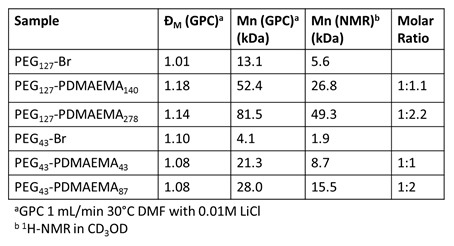
PEG-PDMAEMA diblock copolymers with controlled molecular weight and molecular weight distribution were synthesized using atom transfer radical polymerization (ATRP). ATRP enabled the synthesis of PEG-PDMAEMA diblock copolymers with controlled PEG molecular weight, PDMAEMA molecular weight, and PEG:PDMAEMA molar ratios, as summarized in Table 1. Polymers were characterized by gel permeation chromatography to determine relative molecular weight and molecular weight distribution, and by 1H-NMR spectroscopy to determine the molar ratio of PEG to PDMAEMA. ATRP was used to synthesize a set of polymers with controlled molecular weights, different PEG:PDMAEMA molar ratios, and narrow molecular weight distributions in order to investigate the effect of molecular weight and molar ratios on stimuli-responsive properties.
Table 1. Molecular weight and molecular weight distribution for synthesized PEG-PDMAEMA diblock copolymers.
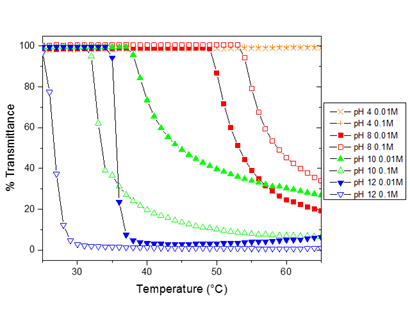
PDMAEMA homopolymers and diblock copolymers change solubility in water due to a change in the balance of intermolecular and intramolecular interactions. At low temperature, PDMAEMA is water-soluble because intermolecular interactions dominate between polymer chains and water. The PDMAEMA chains aggregate as temperature increases because intramolecular interactions overcome the intermolecular hydrogen bonding. The cloud point resulting from a change in solubility can be measured using UV-Visible spectroscopy of aqueous buffered solutions of PEG-PDMAEMA. Transmittance is monitored at 500 nm as a function of temperature and the cloud point is determined from the initial drop in transmittance from 100%.
Figure 2. UV-Vis data for 1 mg/mL PEG127-b-PDMAEMA278 in aqueous buffered solutions.
As seen in Figure 2, both pH and buffer concentration have an effect on PEG-PDMAEMA cloud point. At pH 4, well below the pKa of PDMAEMA, no cloud point is observed. As the pH is increased near the pKa of PDMAEMA, the cloud point decreases with increasing pH. With an increase in pH, the protonation of the PDMAEMA amines decreases, resulting in less repulsion between chains. This decreased repulsion lowers the energy required for the chains to aggregate, resulting in a lower cloud point. For the same polymer concentration, same polymer molecular weight, and same buffer concentration, pH can result in a change in cloud point from 24°C (pH 12, buffer concentration 0.1M) to 54°C (pH 8, buffer concentration 0.1M). Changing buffer concentration changes the ionic strength of the solution, which changes the screening of the PDMAEMA polyelectrolyte. For pH 10 and 12, as expected, increasing the buffer concentration increases the screening and decreases the cloud point. For pH 8, this trend is less pronounced and still under investigation. Additional tests not shown have confirmed that decreasing polymer concentration increases cloud point, and changing either PEG or PDMAEMA molecular weight affects the cloud point.
Personnel Impacts
Funding of this grant provided summer salary for the PI, enabling the mentoring of the undergraduate researchers working on this project. The PI also attended and presented at the national ACS meeting in spring 2015. Preliminary results for this project were obtained using startup funding, and funding of this grant has enabled the expansion of this project. New results obtained on this funded project have been included in a submitted NSF proposal with plans to continue supporting polymer synthesis research with undergraduate researchers.
Three undergraduate researchers at the University of Wisconsin-Eau Claire were supported with this grant. A junior chemistry business major, who obtained the preliminary data that resulted in funding of this grant, was able to continue work on this project in spring 2015. He completed an internship this summer in preparation for obtaining a job upon graduation in spring 2016. Two new undergraduate researchers, a freshman materials science major, and a sophomore chemistry major, started working on this project in spring and summer 2015, respectively. Both were able to work full time this summer on this project, to learn the synthesis and characterization techniques for this project, to achieve great progress on the research, and to gain scientific communication and teamwork skills throughout the summer. In addition to the students funded through this grant, the supply funding enabled the participation of a Brazil Science Mobility Program exchange student in the research progress for the summer. These students have presented their results at the annual university research poster symposium and at state-level research and technology conferences. The sophomore and junior students are continuing work on this project for the next year, they will have the opportunity to present at a national ACS conference, and they plan to pursue careers in materials science or chemistry after earning graduate degrees.