Reports: ND154451-ND1: Catalytic Asymmetric Hydration of Alkenes with Chiral cis-beta Metallosalen Complexes
Mark Rizzacasa, PhD, The University of Melbourne
This project has allowed for the training of one postdoctoral associate and has supported new collaborations both locally and internationally. This project has initiated a new direction of research in my group involving catalysis and the synthesis of chiral octahedral metal complexes and their characterization. Several new chiral catalysts have been synthesized and most have been characterized by NMR and X-ray analysis and these were tested for catalytic activity in Mukaiyama hydration reactions.
Catalyst Synthesis
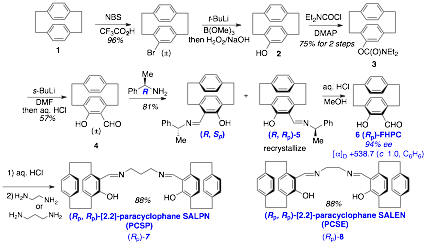
Our initial studies involved the efficient synthesis of the optically pure paracyclophane SALEN and SALPN ligands using a number of literature procedures with some slight modifications.1 As shown in Scheme 1, the synthesis of the ligands began with the bromination of [2,2]paracyclophane followed by installation of the hydroxy group by borylation and oxidative workup (2). Ortho-formylation was difficult, however a reasonable yield was obtained from a 2-step sequence which involved generation of the carbamate 3 followed by treatment with sec-BuLi and DMF. Acid hydrolysis gave the racemic [2,2]paracyclophane salicylate 4. Formation of the imine with (R)-methylbenzyl amine and recrystallisation gave paracyclophane imine 5 in 42% yield and high e.e. after one recrystalization. Hydrolysis of the mother liquor and formation of the imine with (S)-methylbenzyl amine followed by recrystallisation gave the alternative (S, Sp)-paracyclophane imine.
Scheme 1: Synthesis of the paracyclophane ligands (Rp)-7 and (Rp)-8.
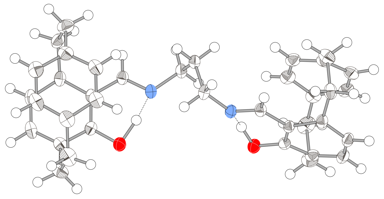
Figure 1: X-ray structure of (Rp)-7
Hydrolysis of the imine 5 with aq. HCl gave the pure (Rp)-FHPC 6 in 94% ee. This was treated with either 1,3-propanediamine or 1,2-ethanediamine to give the respective PCSP (7) and PCSE (8) ligands in good yield. The absolute configuration of the chiral [2,2]paracyclophane derived from the resolution was confirmed as Rp by a single crystal X-ray structure as shown in Figure 2.
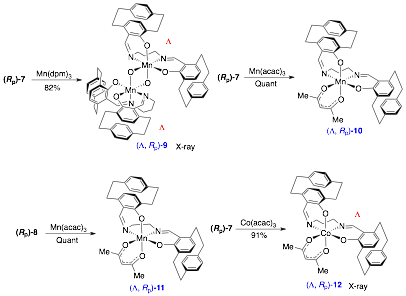
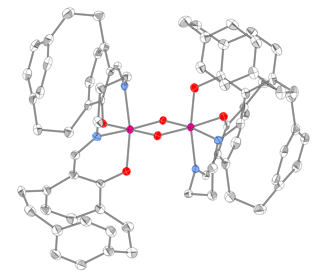
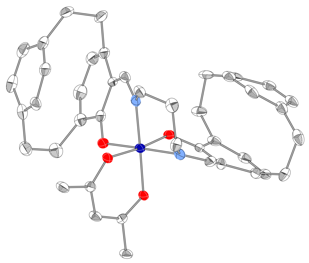
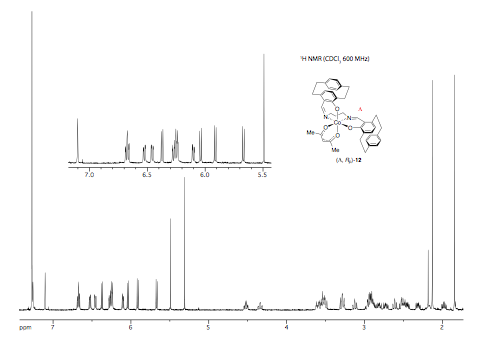
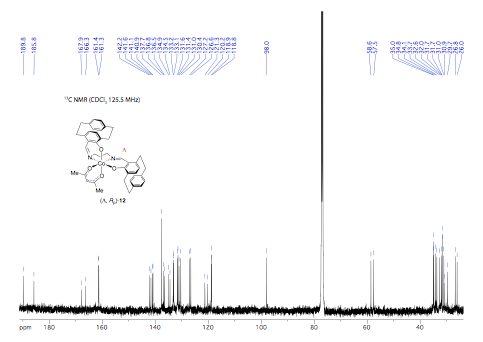
Efforts were then focused on the synthesis of the "chiral at metal" octahedral catalysts (Scheme 2). Ligand (Rp) PCSP (7) was allowed to react with Mn(dpm)3 complex in refluxing methanol and crystals of the product obtained from this reaction were subjected to X-ray analysis. Interestingly, the expected L-cis-b complex did not appear to form as it was oxidised to the subsequent Mn(IV) µ-oxo L, L complex 9 as confirmed by a single crystal X-ray structure as shown in Figure 2. Use of the alternative Mn(acac)3 complex allowed for the preparation of complex (Rp)-10 in high yield but this compound is yet to be fully characterized by X-ray analysis. Ligand (Rp)-PCSE (8) was also reacted with Mn(acac)3 to afford the SALEN complex 11. In addition, reaction of Co(acac)3 with the ligand 7 gave the complex L-12, again the structure confirmed by a single crystal X-ray analysis (Fig 3) as well as NMR spectroscopy (Figs 4 and 5). All the complexes formed as the L isomer only at the chiral metal center as predicted. Thus the ligand (Rp)-7 allows for complete control of the asymmetry at the metal center with the L configuration about the metal.
Scheme 2: Synthesis of catalysts 9, 10, 11 and 12.
Figure 2: X-Ray structure of 9 (H atoms omitted)
Figure 3: X-Ray structure of 12 (H atoms omitted)
Figure 4: 1H NMR spectrum of 12
Figure 5: 13C NMR spectrum of 12
Catalyst Activity
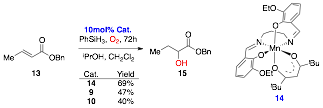
We have tested the reactivity of the new octahedral catalysts using benzyl crotonate (13) as the substrate under the conditions reported2 with catalyst loadings of 10mol% and CH2Cl2 as co-solvent to aid solubility (Scheme 3). The racemic test catalyst 14 was also synthesized to determine if an octahedral Mn(III) complex gave the desired product 15 in 69% yield. The PCSP complexes 9 and 10 also gave the desired product 15 in 47% and 40%. Some starting material remained which could not be consumed either by increased reaction times or adding additional reagents.
Scheme 3: Hydration reactions using catalysts 9, 10, and 14.
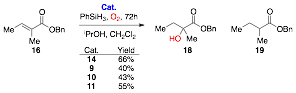
The asymmetric induction was then investigated using tiglate 16 as a substrate in order to give a stable product (Scheme 4). The complex 14 gave the desired product 18 in 66% yield. Both the complexes 10 and 11 gave the desired product along with starting material and a some of the fully reduced compound 19. Carrying the reaction out in the absence of O2 gave the reduction product 19 exclusively, whilst the bubbling O2 through the reaction could eliminate its production. The complex 9 also showed activity, although 8 equivalents of PhSiH3 were required for reasonable yields. Analysis of the derived Mosher esters derived from 18 indicated there was no asymmetric induction. We will continue to develop new chiral octahedral catalysts and investigate a more diverse range of substrates.
Scheme 4: Hydration reactions using catalysts 9-11, 14.
References
1. Belokon, Y.; Moscalenko, M.; Ikonnikov, N.; Yashkina, L.; Antonov, D.; Vorontsov, E.; Rozenberg, V., Tetrahedron Asym. 1997, 8, 3245-3250.
2. Magnus, P.; Payne, A. H.; Waring, M. J.; Scott, D. A.; Lynch, V., Tetrahedron Lett. 2000, 41, 9725-9730.
3. Loits, D.; White, J. M.; Donnelly, P. S.; Rizzacasa, M. A. Unpublished results 2015.