Reports: UR153693-UR1: Intramolecular Cycloaddition Reactions of Electron-Rich Alkynes: New Methods for the Synthesis of Optically Active Building Blocks for Organic Synthesis
Thomas G. Minehan, California State University (Northridge)
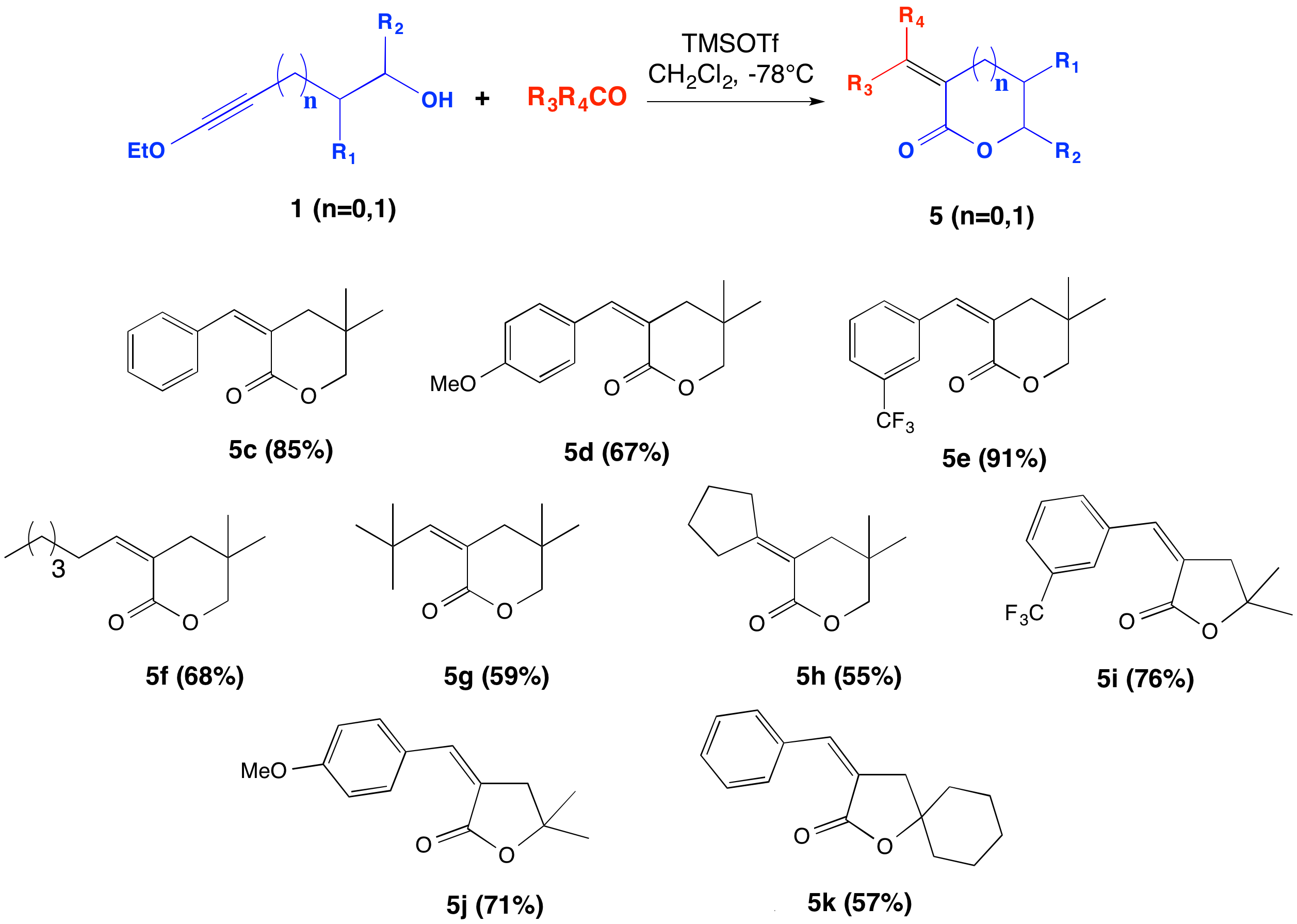
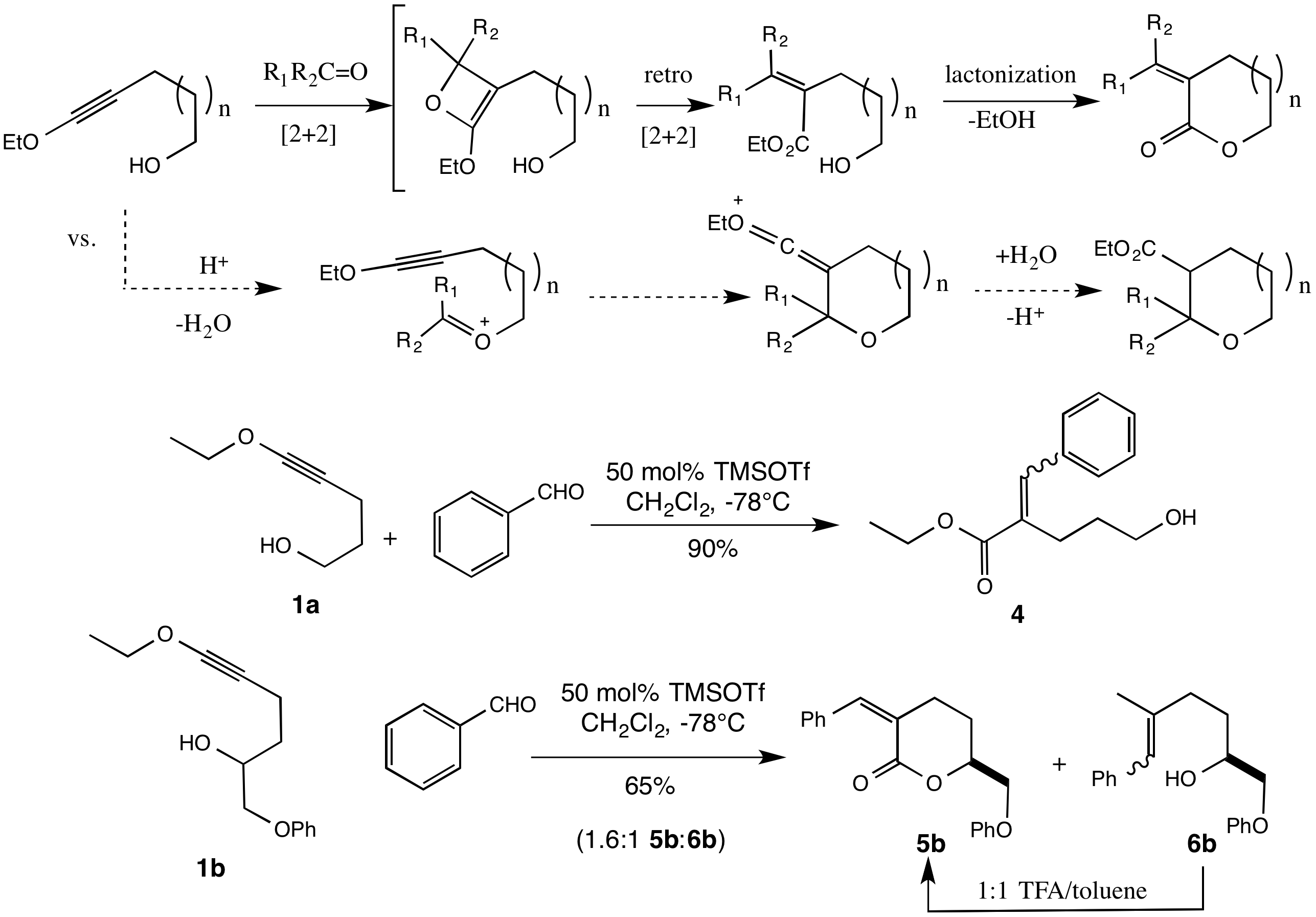
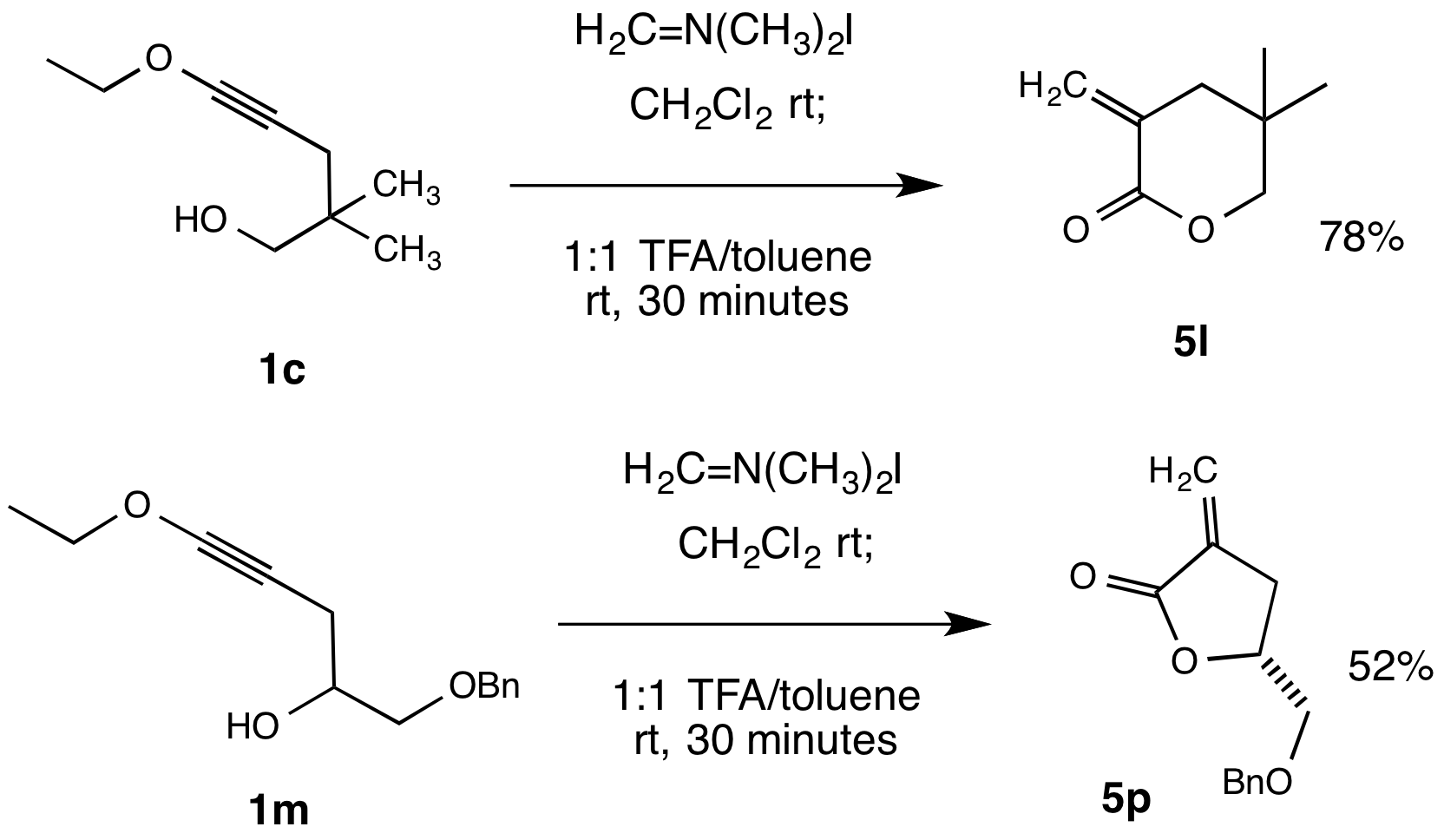
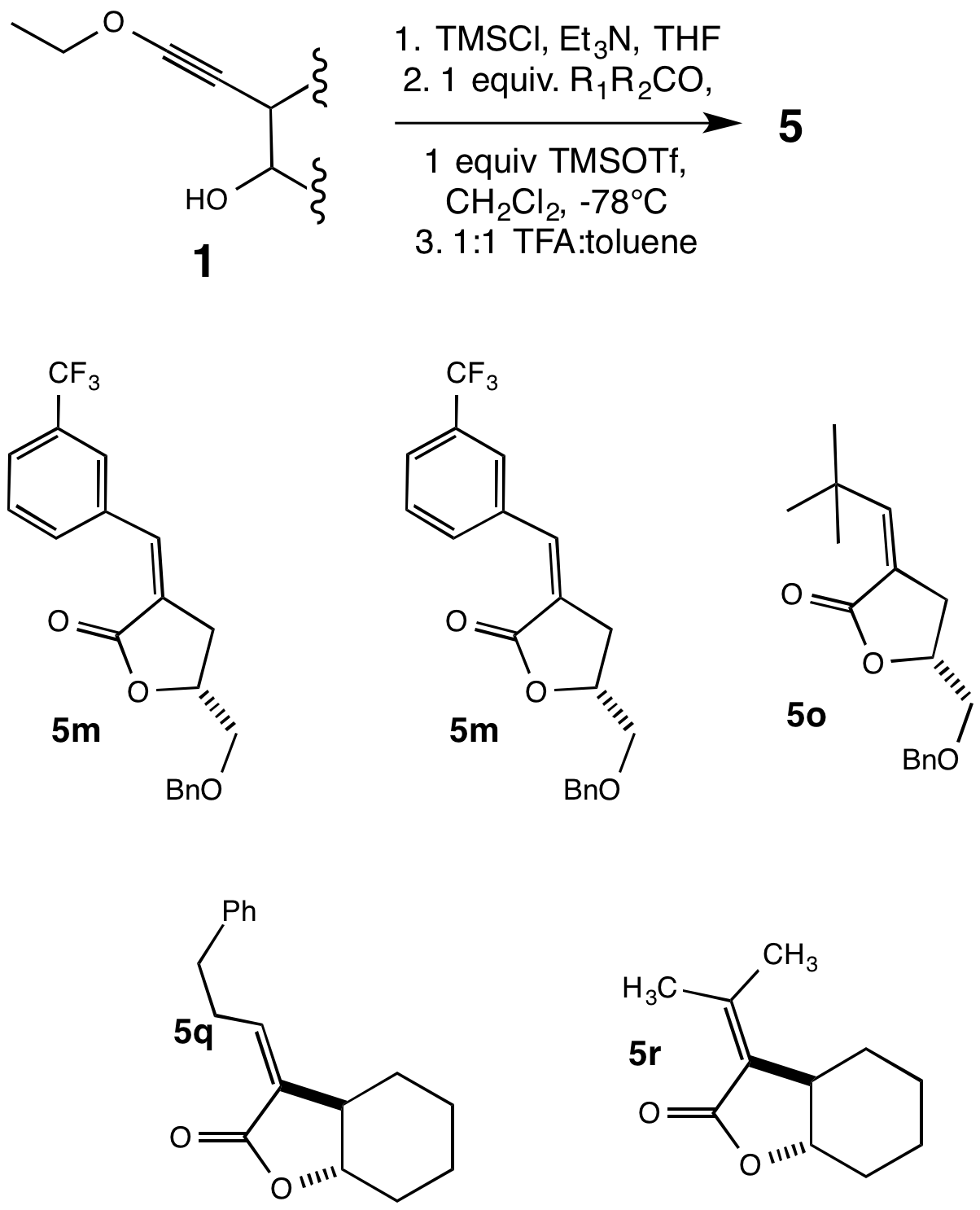
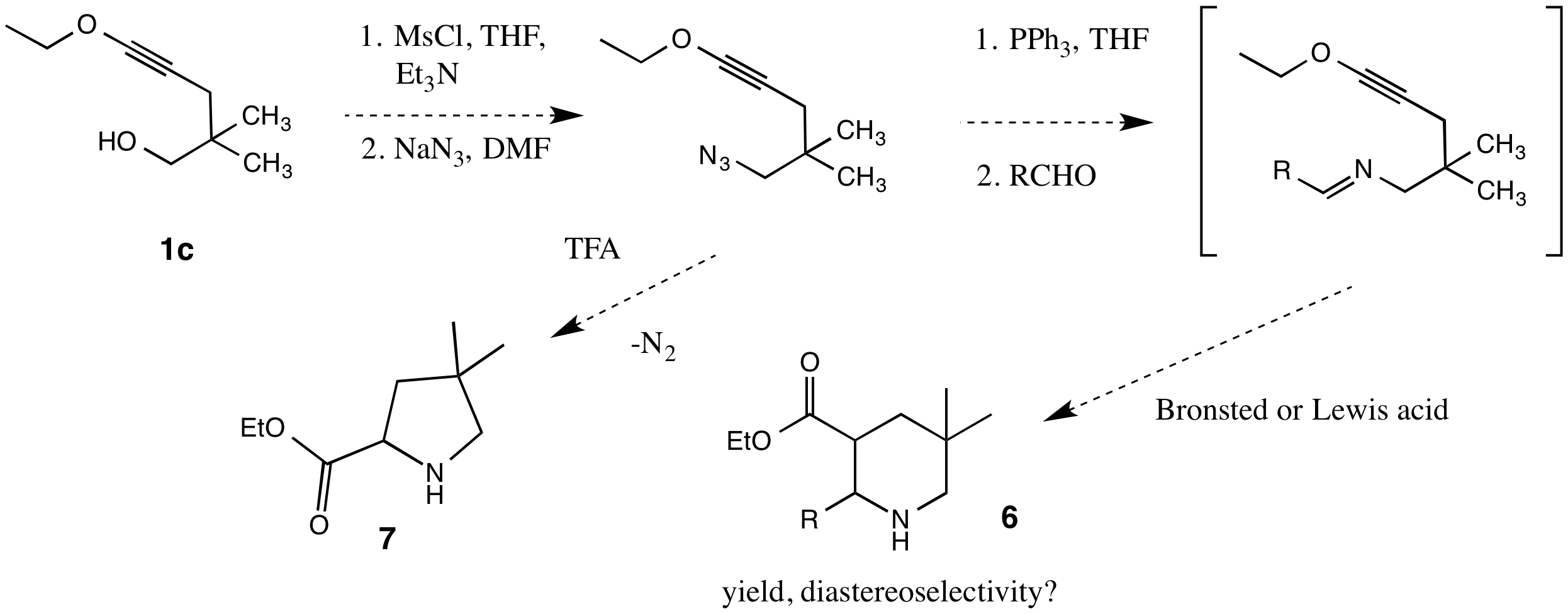
Thomas G. Minehan, California State University (Northridge)





Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society