Reports: DNI154422-DNI1: Cu-Catalyzed Vicinal Dicarbofunctionalization of Simple Alkenes
M. Kevin Brown, Ph.D., Indiana University
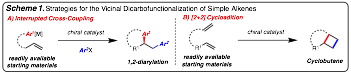
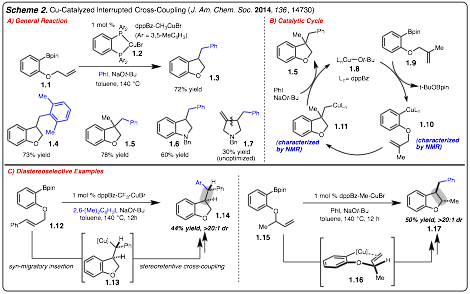

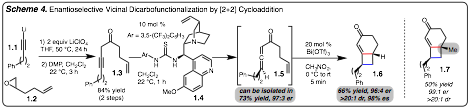
M. Kevin Brown, Ph.D., Indiana University


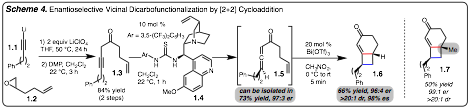
Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society