Reports: UR353234-UR3: Novel Topologically Constrained Transition Metal Complex Oxidation Catalysts
Timothy J. Hubin, Southwestern Oklahoma State University
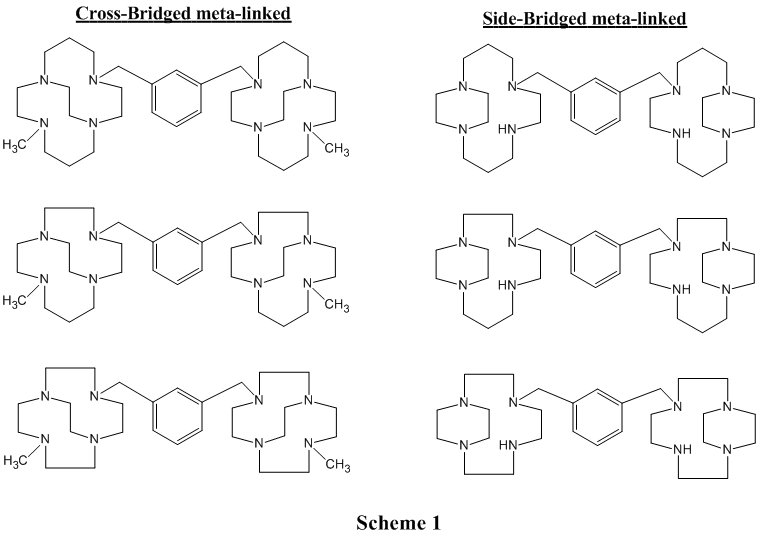
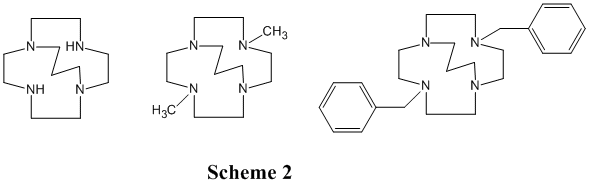
During the grant period for this report we successfully synthesized several ligand targets, including cyclam, cyclen, and homocyclen based meta-linked tetraazamacrocycles containing both ethylene cross-bridges and side-bridges (Scheme 1), and monomacrocyclic cyclen derivatives having a propylene cross-bridged (Scheme 2).
Transition metal complexes, including Fe, Mn, and Cu of most ligands were synthesized successfully, either from the metal chloride or acetate salts. Complete chemical characterization of these complexes in ongoing.
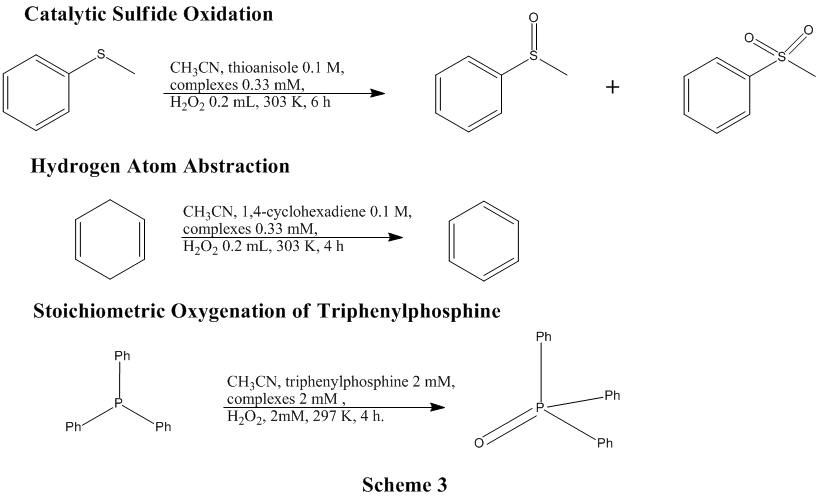
Various catalytic oxidation screening reactions have also been set up with the help of collaborator Guochuan Yin (Scheme 3).
A large group of the metal complexes synthesized have been screened using one or more of these oxidation reactions. Preliminary conclusions about the catalytic oxidation chemistry of these types of complexes include: (1) Manganese complexes are generally slightly more effective catalysts in these ligands than is iron, with copper complexes being much less catalytically active. (2) Bis-macrocycle ligand complexes retain much of the same catalytic activity of their mono-macrocycle derivatives (which are known catalysts), but do not typically work quite as efficiently. (3) Propyl bridged ligands, with only a few tested, do not significantly enhance the catalytic activity versus the more well known ethylene bridged ligand complexes.
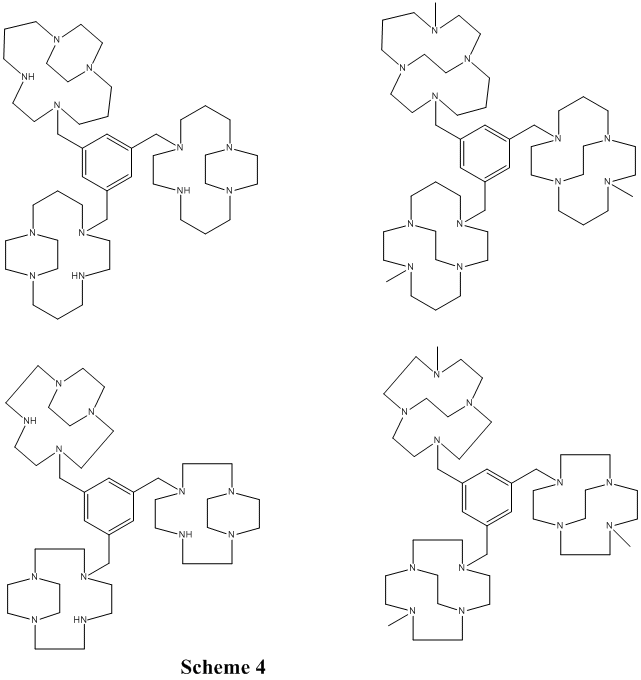
Additionally, a family of C3-symmetryic tris-macrocyle ligands has been synthesized and characterized (Scheme 4) and their transition metal chemistry is beginning to be explored. We have not yet screened any of these complexes for oxidation catalysis.
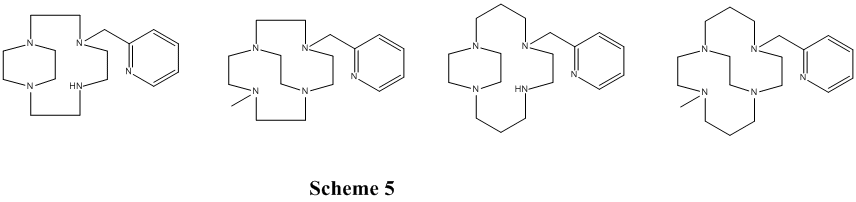
Finally, a set of pyridyl pendant arm side- and cross-bridged ligands and complexes have been synthesized and the cyclam cross-bridged derivatives found to have active, selective, catalytic oxidation properties. (Scheme 5)