Reports: DNI152073-DNI1: Development of Novel, Stable and User-Friendly Trifluoromethylation Reagents
Ryan A. Altman, PhD, University of Kansas
Overview
In the last year, PRF DNI-supported research has focused on the conversion of alcohols into fluoroalkyl analogs. These strategies enable the preparation of new fluorinated compounds from common alcohol-based petroleum reformats.
Fluorinated functional groups, including the trifluoromethyl (CF3) and a,a-difluoroketone groups, serve important roles in medicinal, agrochemical and materials chemistry by perturbing the (bio)physical properties of the parent molecule. Thus, the development of new chemicals methods to install fluorinated groups is critical for accessing new therapeutics, biological probes, agrochemical agents, and materials. Although many creative and useful metal-catalyzed trifluoromethylation reactions have been developed, certain simple and desired transformations have not been achieved. One such gap involves the conversion of readily available alcohol-based functional groups into trifluoromethyl and a,a-difluoroketone analogs.
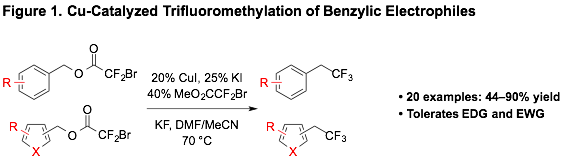
Cu-Catalyzed Trifluoromethylation of Reactions of Bromodifluoroacetic Esters
Among CF3-containing substructures, trifluoroethyl(hetero)arenes represent an important motif, with over 30,000 trifluoroethyl(hetero)arenes possessing documented biological activity or being precursors to bioactive compounds. Thus, general strategies for preparing this substructure are important for accessing biological probes and therapeutics. Further, strategies that also involve reactions of alcohol-based petroleum reformats align with the goals of the PRF program. Trifluoroethylarenes can be directly accessed via nucleophilic trifluoromethylation of benzylic electrophiles; however, current catalytic methods do not effectively transform electron-deficient substrates and heterocycles.
To address this gap, we developed a Cu-catalyzed decarboxylative trifluoromethylation of benzylic bromodifluoroacetates (Figure 1). Two key features, solvent and I–, enabled a Cu-catalyzed decarboxylative trifluoromethylation of benzylic and heterobenzyl bromodifluoroacetates. The protocol transformed a variety of benzylic bromodifluoroacetates, including electron-deficient and heterocyclic substrates, and substrates bearing carbonyl groups and acidic protons. The expanded functional group compatibility was rationalized by a metal-centered decarboxylation event, which did not seem to generate free –CF3 in solution. We envision that this system will be useful for accessing biological probes, therapeutic agents, and agrochemicals from simple petroleum-derived alcohols.
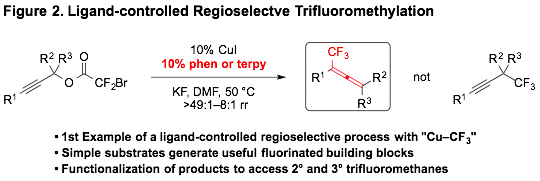
Additionally, Cu-catalyzed reactions were developed for converting propargyl bromodifluoroacetates into trifluoromethyl allenes (Figure 2). Cu–CF3 species have been used historically for a broad spectrum of nucleophilic trifluoromethylation reactions. Although recent advancements have employed ligands to stabilize this key organometallic intermediate, the ability of a ligand to differentiate the regiochemical outcome of a Cu−CF3-mediated or -catalyzed reaction had not been previously reported.
We developed the first example of a Cu-catalyzed trifluoromethylation reaction for which a ligand controls the regiochemical outcome. More specifically, the use of bipyridyl-derived ligands enabled a Cu-catalyzed nucleophilic trifluoromethylation reaction of propargyl electrophiles to generate (trifluoromethyl)allenes. This method provided a variety of di-, tri-, and tetrasubstituted (trifluoromethyl)allenes, which can be further modified to generate complex fluorinated substructures.
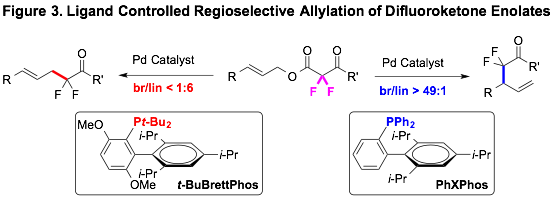
Pd-Catalyzed Decarboxylative Allylation and Benzylation Reactions of Difluoroketone Enolates
a,a-Difluoroketones possess unique physicochemical properties that are useful for developing therapeutics and probes for chemical biology. A convergent preparation of this substructure would involve a transformation capable of generating the C(a)–C(sp3) bond, presumably by reacting an a,a-difluoroenolate enolate with an sp3-hybridized allylic electrophile. Alkylation of ketone enolates with sp3-based electrophiles via SN1 and SN2 is a fundamental transformation for accessing a broad spectrum of α-functionalized ketones; however, direct alkylation reactions of weakly nucleophilic a,a-difluoroketone enolates with sp3-hybridized electrophiles remain nearly unexplored, even for activated allylic electrophiles. To access the a-allyl-a,a-difluoroketone substructure, complementary palladium-catalyzed decarboxylative allylation reactions were developed, which provide both linear and branched a-allyl-a,a-difluoroketones. For these orthogonal processes, the fluorination pattern of the substrate enabled the ligands to dictate the regioselectivity of the transformations. The reactions provided convergent access to a-allyl-a,a-difluoroketone-based products, and should be useful for accessing biological probes directly from alcohol-based petroleum reformats.
Impact of PRF DNI Award
The PRF DNI award has positively affected Dr. Altman's career, as well as multiple group members involved in the project. This award has provided training for postdoctoral research assistants and graduate students in the fields of synthetic organic, organofluorine, and organometallic chemistries. Specifically, the researchers were intimately involved in developing the chemical methods and conducting synthetic chemistry to prepare substrates. Most notably, the researchers were challenged to think critically to solve chemical problems, and to probe the mechanistic underpinnings of the transformations. The support provided by the PRF award has positively impacted the research group by providing an opportunity to pursue basic chemical research. Further, the results obtained from PRF support will continue to drive an area of research in Dr. Altman's group for years to come, which will provide valuable synthetic tools for accessing new fluorinated analogs of compounds directly from petroleum reformats. The results described in this report provide a solid foundation for ongoing and future work in the group that aims to develop innovative decarboxylative fluorination reactions for accessing fluorinated compounds that have practical applications within many chemical sub disciplines.