


|

|

|
Suzanne Blum, Elizabeth Jarvo, and Chris Vanderwal have a few things in common. They are all organic chemists, professors at the University of California Irvine, and deeply committed to their areas of study. They all credit ACS PRF for launching their current research, early on in their careers. Dr. Jarvo describes the role of ACS PRF as fundamental - it was the first grant that I received and it started this project.
Dr. Blum agrees. "The idea that ACS PRF is willing to fund very early, very speculative work has been really great for us."
Though situated under the main umbrella of organic chemistry, the three colleagues do focus on different topics. Jarvo offers up this explanation. "Imagine a line that runs from fundamental organometallic chemistry to actually building complex molecules for use in pharmaceuticals," she instructs. "We're all sort of on this timeline. Blum falls earliest on this line, doing research on the most basic organometallic chemistry. Jarvo falls next on the continuum, and then Vanderwal, who deals with synthesis of complex molecules. "There's a nice synergy," Jarvo adds. "If you're going to make new molecules, you need a new reaction to be able to do it with. If you're going to build a new reaction, you need someone to show that it's actually useful for molecular synthesis."
Blum's group studies the building blocks of fundamental chemical reactivity, specifically catalysts. Catalysts are small amounts of a chemical that increase the rate of a chemical reaction. "What my group is doing that is different from previous work in the field, is developing reactions with two catalysts instead of one," she says. Thus far, Blum has used this concept to develop over three distinct new catalytic reactions. The chemical products produced from these reactions are made in a more efficient and controlled manner, with fewer byproducts.
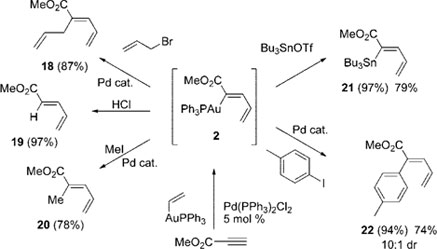
Scheme taken from Dr. Blum's research
Jarvo's area of research is slightly different from Blum's. She also studies chemical reactions, though with the goal of taking molecules in petroleum byproducts and turning them into other, more useful molecules, such as those used in preparing pharmaceutical agents and chemicals. The method involves employing an allylation reaction to achieve the transformation, and organometallic compounds as the method of catalysis, in order to efficiently enable the production of new molecules.
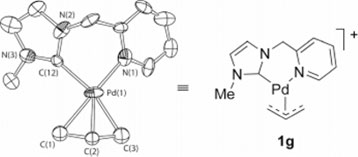
Scheme taken from Dr. Jarvo's research
Vanderwal and his team are situated a bit further down the timeline, studying how to create applications for the synthesis of complex natural products. They focus on forming complex molecules from simple molecular starting materials. "What we've done is take a class of molecules called pyridines, which are inexpensive, and converted them into a variety of molecules that would be much more difficult to access before our chemistry came along." The product molecules are (non-pyridine) heterocycles, including indoles, which are prevalent in many naturally-occurring antifungal and anticancer compounds, as well as other pharmaceutical agents. The ultimate goal of this molecular tailoring is to benefit science and medicine by enabling access to better, cheaper, and more efficient drugs. "Basically, everything we do revolves around making organic chemistry more efficient," Vanderwal states.
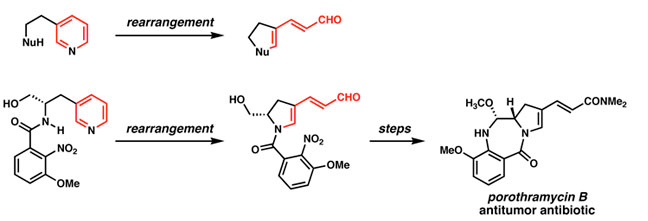
Scheme taken from Dr. Vanderwal's research
Blum and Jarvo, too, see long-ranging important ramifications for their work. Jarvo finds it particularly rewarding that there have been four or five new projects that have spun off from her original PRF-funded research. "It started a chain reaction of sorts," she says. "It's great to look back and think about how much we've evolved."
Continue reading about Dr Blum's study of Dual Catalytic Reactions, Dr. Jarvo's research on Palladium-based Catalysis for Allylation Reactions of Aldehydes, and Dr. Vanderwal's work with the Synthesis of Heterocycles by the Ring Opening of Pyridinium Salts.