43673-AC1
Improving Turnover Numbers of Palladium N-Heterocyclic Carbene Catalysts in Challenging Processes: Intramolecular Direct Arylation Reactions with Aryl Chlorides
In the past decade, direct arylation has emerged as an increasingly viable alternative to traditional cross-coupling techniques. A number of arenes have been shown to participate in these transformations, including electron-rich and electron-deficient heterocycles, as well as simple benzenes.
Our original proposal outlined four goals: (1) the study N-heterocyclic carbene palladium catalysts in intramolecular direct arylation reactions with aryl chlorides; (2) understand the mechanism(s) of catalyst death, and discover method(s) to either prevent or circumvent it (them); (3) investigate discoveries achieved in the first two pursuits in the context of other important palladium-catalyzed cross-coupling reactions; and (4) conduct a total synthesis of an important anti-tumour agent, allocolchicine. Over the course of this grant, Objectives 1, 2, and 4 were completed in entirety. Goal #3 is open ended, and this is where the last year of the grant cycle was dedicated. These advances have lead to the publication of one manuscript, should result in the publication of one more in the upcoming year, and have resulted in the body of knowledge described below.
Azaindoles, which possess the same (4.3)-bicyclic indene architecture as indoles but with a second nitrogen in the azine ring, have demonstrated importance in medicinal chemistry. Compared to indoles, however, they are far more challenging to prepare, especially in highly functionalized form. In the context of direct arylation, a C2 arylation of 7-azaindole has been described, but methods for the direct functionalization of the azine ring have not been forthcoming. Through these studies, we found that by employing the N-oxide azine activation strategy, both 7- and 6-azaindoles will undergo regioselective direct arylation of the azine ring in synthetically useful yields. We have also found that by modifying the Larrosa arylation protocol by heating to 80 degrees C, that highly selective C2 arylation can be induced, offering a divergent method for the preparation of polyaromatic compounds based on an azaindole core.
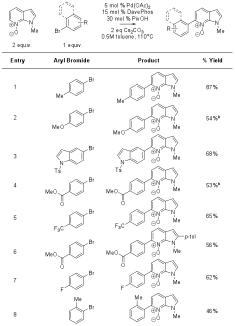
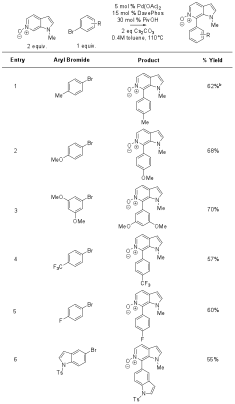
Illustrative examples of the scope for C6 arylation of N-methyl-7-azaindole N-oxide (5) are included in Table 1. Although substrates derived from 4- and 5-azaindole (1 and 2) displayed minimal reactivity under the optimized conditions, N-methyl-6-azaindole N-oxide (12) and p-tolyl bromide (6) reacted to give the C7 cross-coupled product in 62% yield (Table 2, entry 1). Additional examples of the scope with the 6-azaindole substrate are included in Table 2.
Table 1. Scope of N-methyl-7-azaindole N-oxide (5) azine arylation.a
a Conditions: aryl halide (1 equiv.), N-oxide (2 equiv.), Cs2CO3
(2 equiv.), Pd(OAc)2 (5 mol %), DavePhos (15 mol %), and PivOH (30
mol %) were weighed into a vial, purged with argon, charged with toluene (0.5
M), and stirred at 110�C overnight. b
3 equivalents of N-oxide used. Table 2. Scope of N-methyl-6-azaindole
N-oxide (12) azine arylation.a
a Conditions: aryl halide (1 equiv.), N-oxide (2 equiv.), Cs2CO3
(2 equiv.), Pd(OAc)2 (5 mol %), DavePhos (15 mol %), and PivOH (30
mol %) were weighed into a vial, purged with argon, charged with toluene (0.4
M), and stirred at 110�C overnight. b
No PivOH added. We then evaluated the arylation of the azole ring of
azaindoles. Employing N-methyl
7-azaindole as coupling partner, we examined the use of palladium(II) catalysts
in conjunction with boronic acids, palladium(0) catalysts with aryl iodides,
and palladium(II) or copper(II) catalysts with iodine(III) reagents. While the
conditions of Larrosa initially provided only 6% conversion by GCMS, a survey
of the reaction components revealed that upon heating the reaction mixture to
80�C,
arylation of N-methyl-7-azaindole was
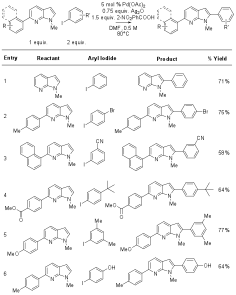
acheived in 71% isolated yield with complete C2 regioselectivity (Table 3,
entry 1). Illustrative examples of
the scope for this transformaiton are included in Table 3. Table 3. C-2 arylation of 7-azaindoles.a
a Conditions: Azaindole (1 equiv.), aryl iodide (2
equiv.), Pd(OAc)2 (5 mol %), silver(I) oxide (0.75 equiv), and
2-nitrobenzoic acid (1.5 equiv.) were weighed into a vial, purged with argon,
charged with DMF (0.5 M), and stirred at 80�C overnight. During the last stage of this
research proposal, we have demonstrated arylation of the azine ring of N-methyl 7- and 6-azaindoles by N-oxidation and subsequent direct
arylation with aryl bromides. We have also established that following N-oxide reduction, subsequent arylation
of the azole ring of 7-azaindole substrates can be effected in good yield.