


| Theoretical Investigation of Multireference Diradical Systems |
"Often, a chemist will mix two things together and they'll get a reaction," says Carol Parish, a professor in the Department of Chemistry at the University of Richmond, "but it's not always possible to unambiguously understand everything that's happening at the atomic level." She pauses. "That was frustrating to me."
Professor Parish explains how she turned to theoretical computational chemistry in order to satisfy her growing fascination with molecular behavior. "I wanted to have some confidence in my ability to predict the future behavior of a molecule. And in order to do that, I had to understand the current behavior of a molecule."
Parish, who typically leads a team ranging anywhere from 10 to 15 undergraduate students, is attempting to do just that. By employing the laws of math and physics, she and her team can create highly accurate computer models to simulate molecular behavior. They are in the process of exploring a variety of systems, including fluorescent biosensors, molecular scaffolds for drug delivery, excited states of possible anticancer drugs, and annulenic molecules that show potential as optical materials.

Dr. Parish's 2009 student team
While Parish defines her work as "diverse by design," the unifying theme for all of her seemingly disparate research is the methodological approach she employs. She likens it to a "divide and conquer" strategy. She solves challenging problems by breaking them down into smaller, easily grasped pieces that clearly illustrate fundamental properties. What sets her team apart from the multitude of other researchers working on molecular behavior modeling is the vast array of options in their scientific toolbelt. "We know how to properly use many diverse tools, so that we can focus on carefully choosing the right theoretical method to solve any problem at hand," she states.
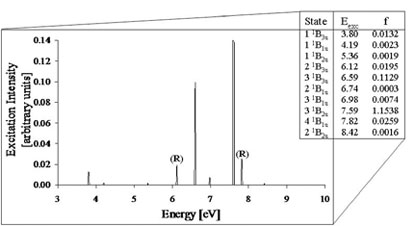
Figure taken from Dr. Parish's research
Most recently, Parish has begun focusing on diradical chemistry, the chemistry of molecules with unpaired electrons, a topic which has become increasingly important in our energy-hungry world. Knowledge of diradical molecules, she says, will help us to understand the reactivity in combustion chemistry of untapped hydrocarbon reserves. In less accessible oil sand and oil shale deposits in the United States, where oil is tied up in sand or bio-materials, understanding the molecular nature and behavior of the constituent species will help motivate further prospecting and exploration of these untapped resources.
The opportunity to engage in projects that produce meaningful results appeals to Parish, both as an educator and as a research scientist. She points out that it is the combined power of these two roles that gives her the most satisfaction, allowing her to set ambitious research goals in addition to inspiring the next generation of chemists on a daily basis.
Read more about Dr. Parish's research, A Computational Investigation of Molecular Recognition and Binding Free Energies in Host-Guest Systems.