
Back to Table of Contents
45095-G1
A Novel Synthesis of Nitrogen Heterocycles by Ring-Opening Reactions of Pyridinium Salts
Christopher D. Vanderwal, University of California (Irvine)
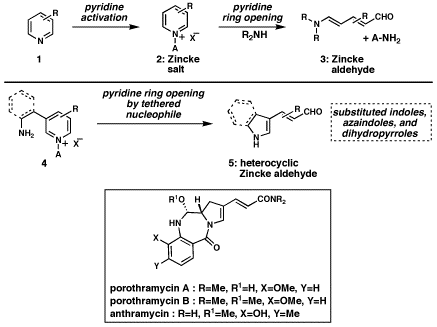
The ring opening
reaction of pyridinium salts dates back over a century to the pioneering work
of Zincke and Kšnig. Treatment of appropriately activated pyridinium salts such
as 2
(Figure 1) with secondary amines cleanly affords the products of ring opening.
The product 5-amino-2,4-pentadienals (3), now known as Zincke aldehydes, appear
ideally suited for manifold applications in synthesis; to date, however, this
potential has remained largely unrealized.In the research funded
by our grant from the American Chemical Society's Petroleum Research Fund, we
have found that tethering nitrogen-based nucleophiles to the 3-position of the
pyridine enables a new heterocycle synthesis (see 4 to 5). After activation of
the pyridine nucleus and cyclization of the tethered nucleophile onto C2 of the
electrophilic pyridinium salt, which induces pyridine ring-opening, new
non-pyridine heterocycles with integrated Zincke aldehyde motifs result. This
new reaction was primarily applied to the synthesis of a variety of substituted
indoles, that each bear the versatile propenal functional group appended to C3
of the indole. A variety of substituents are tolerated, and even azaindoles can
be made in this way. Finally, as a model study for an eventual synthesis of the
porothramycin/ anthramycin family of antitumor antibiotics, an N-benzoylated
dihydropyrrole was synthesized, which corresponds to the central core of these
compounds. In the last year of our support from ACS–PRF, we have
published these initial results as a Communication in Angewandte Chemie. We have also recently
made excellent progress in adapting our published synthesis of N-benzoylated
dihydropyrroles to the synthesis of the porothramycins; we anticipate
completion of this synthesis and publication by the end of 2008.The use of the century old pyridinium ring opening
reaction with tethered nucleophiles has led to a convergent two-step synthesis
of a series of indole-3-propenals. Preliminary experiments with tethered amides
have indicated that with the correct choice of experimental parameters,
variation of nucleophile/tether combinations is also tolerated. Therefore, this
reaction of pyridinium salts represents not simply a new indole synthesis, but
rather the first step towards a potentially general protocol for heterocycle
synthesis. We envision extension to oxygen- and sulfur-containing heterocycles,
and carbocycles, as well as applications in natural product synthesis and
medicinal chemistry.
Back to top


