46807-G4
Controlled Electron Transfer in Hydrogen Bonded Systems
The aim of our research is to improve the effectiveness of photoinduced charge-separation between donors and acceptors. Since the efficiency of this process is tightly correlated with the performance of solar cells, we expect this work to provide a basic knowledge for the improvement of photovoltaic devices. In this specific project, we investigated hydrogen-bonded naphthalimide-pyridine (NI-PYR) systems, with a goal to learn how to take advantage of H-bond dynamics to control the electron flow in molecular systems. The NI-PYR derivatives were designed in such a way so that the initial photoinduced electron transfer from NI to PYR triggers a proton motion along an H-bonded surface. This in turn is expected to make the charge recombination thermodynamically and/or kinetically inefficient. In other words, the H-bonded surface could act as a unidirectional gate for the electron flow.
To obtain a good understanding of the chromophore used in our donor-acceptor system, we started out with the investigation of excited state properties of five NI derivatives.1 The study involved synthesis and solvent-dependent absorption and emission spectra. Furthermore, the excited state dynamics of these compounds were analyzed using femtosecond optical and mid-IR pump-probe spectroscopy. While the optical pump-probe setup has already been available as a part of our shared laser facility here at BGSU, my group has built a transient absorption setup that can probe in the mid-IR range. To obtain a better understanding of our experimental results, we started collaboration with Prof. Christopher Hadad, who characterized the excited state behavior of NI derivatives using DFT.
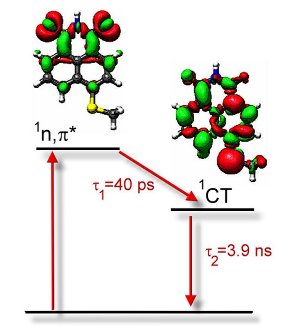
One of the main
findings of our work is that the excited state properties of NI derivatives are
characterized by a competition between n,
The information about the
character of these two states was obtained from DFT calculations. The above
figure presents difference density plots of S2 and S1
states in SMe-NI. In the case of S2 excited state, electronic charge
is depleted from lone electron pairs of imide oxygen atoms and a charge is
accumulated at the aromatic rings. This sort of difference density plot is characteristic
of an n,
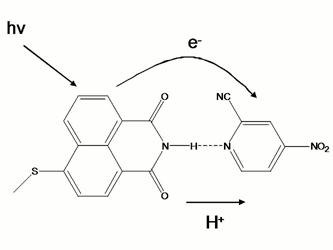
We further studied
electron transfer dynamics from excited SMe-NI to NO2,CN-PYR.2
The donor-acceptor system was chosen in such a way to enable: (i) photoinduced
electron transfer from NI to PYR and (ii) proton transfer from NI radical
cation to PYR radical anion that are produced upon electron transfer. The main
finding of our work is that the fast deactivation of the excited state occurs
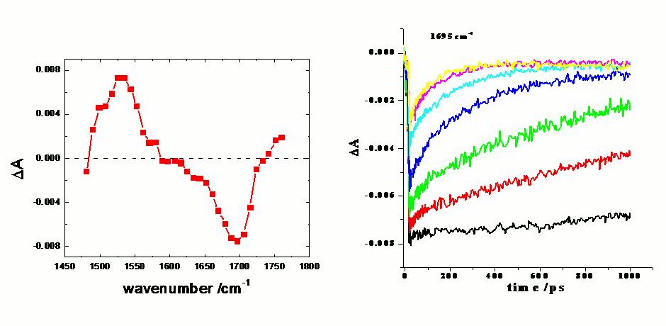
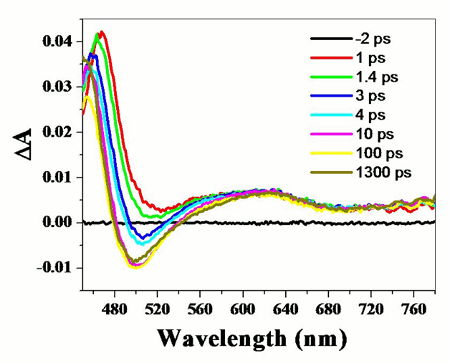
in the H-bonded complex. This effect can be best observed from the figure above,
which presents the dynamics of bleach recovery of the SMe-NI carbonyl-group at
1695 cm-1. As the concentration of the PYR is increased, less
excited state absorption is observed at t=0, suggesting that the deactivation
of the NI excited state in H-bonded complex is faster than the time-resolution
of our instrument (~300 fs).
Unfortunately,
the fast thermal deactivation of the SMe-NI excited state in H-bonded complex
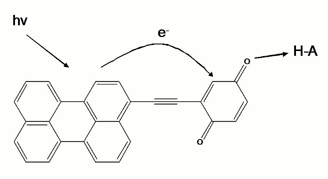
made it impossible to study electron transfer dynamics in NI-PYR systems. To
overcome this problem, we initiated a preparation of covalently bound donor-acceptor
system presented in the scheme above. We are currently in the last step of
synthesis, and we expect this model compound to be better for electron transfer
studies.
Reference:
(1) Pavel
Kucheryavy, G. L., Shubham Vyas, Christopher Hadad, Ksenija D. Glusac,
"Excited State Properties of 4-Substituted Naphthalimides", In Preparation.
(2) Pavel Kucheryavy, G. L., Shubham Vyas,
Christopher Hadad, Ksenija D. Glusac, "Ultrafast Excited State
Deactivation in Hydrogen-Bonded Naphthalimide-Pyridine Donor-Acceptor
Systems", In Preparation.