Reports: ND353839-ND3: Dinitrogen Reduction by Pincer Complexes of Early Transition Metals
Gregory S. Girolami, PhD, University of Illinois (Urbana-Champaign)
The goal of this project is to develop new complexes of the early transition metals bearing P-C-P pincer ligands, with a particular focus on low valent metal complexes that may provide new opportunities for the activation of small molecules such as dinitrogen. In this report we give the results of the first several months of this project.
The P-C-P pincer ligand 2,6-bis((di-t-butylphosphino)methyl)phenyl lithium (Li(tBuPCP)) was prepared by modifying literature procedures for similar compounds.1-3 The reaction of this compound with one equivalent of TiCl3(thf)3 in pentane affords the novel compound (tBuPCP)TiCl2, which crystallizes as blue-violet prisms from pentane. The titanium centers in (tBuPCP)TiCl2 have a distorted square pyramidal geometry in which one of the chloride atoms, Cl(1), occupies the axial site (Figure 1).
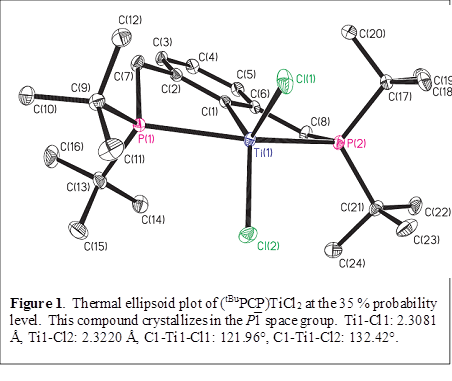
As a result of the opening up of the C-Ti-Cl(2) angle, the two C-Ti-Cl angles of 122.0° and 132.4° differ by 10.4°. As expected, the Ti-Cl bond that is more trans to the aryl carbon atom is slightly longer.
The titanium(III) complex (tBuPCP)TiCl2 reacts with two equivalents of LiBH4 in Et2O to generate the bis(borohydride) analog (tBuPCP)Ti(BH4)2, which crystallizes as purple prisms from diethyl ether. The metal centers in (tBuPCP)Ti(BH4)2 adopt square pyramidal geometries about the Ti atom (Figure 2), but basal C-Ti-B angle is not opened up as much, so that the geometry is not far from trigonal bipyramidal, with the phosphorus atoms in the two axial sites.
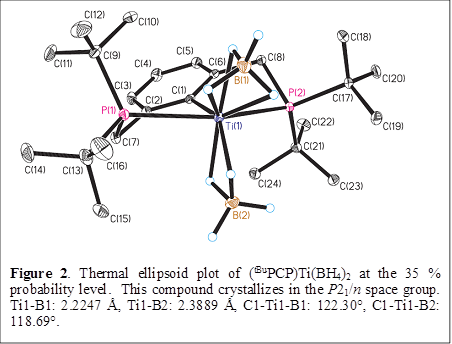
The two C-Ti-B angles of 118.7 and 122.3° differ by only 3.6°. One of the BH4- ligands in (tBuPCP)Ti(BH4)2 is κ2 whereas the other is κ3. This assignment is corroborated by the Ti-B distances as well as by the IR spectrum, which gives a pattern in the B-H stretching region that is similar to those of other known κ2, κ3 bis(borohydride) compounds. The κ2, κ3 structure can be rationalized on electronic grounds: of the nine valence orbitals available (s + 3p + 5d), three are used to form sigma bonds with the coordinating atoms of the P-C-P ligand, and one is used to house the single d-electron, so that five orbitals are available for Ti-H bonding to the borohydride ligands.
Reactions of the chloro complex (tBuPCP)TiCl2 with alkyllithium reagents and with N2 in the presence of a reductant are currently under investigation.
Reaction of Li(tBuPCP) with CrCl3(thf)3 in Et2O, followed by crystallization from thf, affords the chromium(III) pincer compound (tBuPCP)CrCl2(thf). The Cr center in in this compound is octahedral (as expected), with the thf ligand trans to the aryl carbon atom (Figure 4).
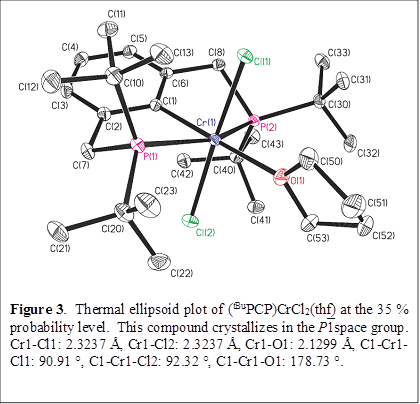
Trituration with pentane or heating to 60 °C under vacuum converts this compound into the solvent-free analog (tBuPCP)CrCl2. Desolvation causes a relatively significant color change from blue to orange.
Treatment of the chromium(III) compound (tBuPCP)CrCl2 with excess KC8 in Et2O can be causes a reduction to the chromium(II) species (tBuPCP)CrCl, which crystallizes as violet prisms from Et2O. The chromium center in (tBuPCP)CrCl is square planar, as is often the case for high-spin d4 first-row metal complexes (Figure 4).
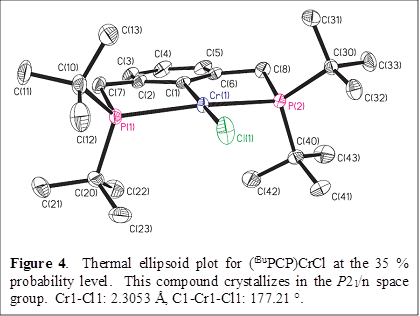
Reactions of this latter complex are currently being investigated.
One of the issues we have encountered in this work is that the tert-butyl substituted pincer ligand gives complexes that can sometimes be difficult to crystallize owing to their high solubility. In an effort to modify the chemistry so that that crystallization occurs more readily, we have recently begun an investigation of the analogous adamantyl-substituted pincer ligand 2,6-bis((di-1-adamantyl)phosphino)methyl)phenyl (AdPCP). Efforts to prepare Li(AdPCP) by straightforward extension of the route that successfully affords Li(tBuPCP) have not been successful, a fact that we attribute to the kinetic effects caused by the larger steric demand of the adamantyl groups. We have, however, been able to make progress toward this goal by more significant modifications of the synthetic procedure. Two equivalents of the secondary phosphine Ad2PH, prepared according to at literature route,4 and one equivalent of 2-bromo-1,3-di(bromomethyl)benzene, when heated to reflux in the presence of excess NEt3, afford the bromo precursor Br(AdPCP) in approximately 60 % yield. The lithiation of this compound is currently being studied.
The PRF grant has enabled graduate students in my group to learn air-sensitive synthesis techniques, as well as methods of characterization such as NMR spectroscopy and X-ray crystallography.
References
1. Shim, S. C.; Chae, S. A.; Lee, D. Y.; Youn, Y. Z.; Shim, J. G.; Doh, C. H., "Carbonylation of 1-Bromo-2,6-Bis(Bromomethyl)Benzene Catalyzed by Cobalt Carbonyl." Bull. Korean Chem. Soc. 1993, 14 (4), 481-485.
2. Montag, M.; Schwartsburd, L.; Cohen, R.; Leitus, G.; Ben-David, Y.; Martin, J. M. L.; Milstein, D., "The Unexpected Role of CO in C-H Oxidative Addition by a Cationic Rhodium(I) Complex." Angew. Chem., Int. Ed. 2007, 46 (11), 1901-1904.
3. Pape, A.; Lutz, M.; Muller, G., "Phosphane Coordination to Magnesium - Synthesis and Structure of Bis[Ortho,Ortho'-Bis((Dimethylphosphino)Methyl)Phenyl]Magnesium." Angew. Chem., Int. Ed. 1994, 33 (22), 2281-2284.
4. Goerlich, J. R.; Schmutzler, R., "Organophosphorus Compounds with Tertiary Alkyl Substituents. VI: A Convenient Method for the Preparation of Di-1-Adamantylphosphine and Di-1-Adamantylchlorophosphine." Phosphorus, Sulfur Silicon Relat. Elem. 1995, 102 (1-4), 211-215.











