Reports: UNI153730-UNI1: Synthesis of Highly Substituted Piperidines from Petrochemical Compounds
Jenny M. Baxter Vu, PhD, Valdosta State University
Introduction
The construction of complex, versatile molecules from unsaturated hydrocarbons, abundant in petrochemical feedstock, is highly desirable. While cycloaddition reactions of 1,3-dipolarophiles with dienes has also been reported in the literature, the full potential of this transformation has not been exploited. Our initial focus was on the [3+2] cycloaddition reaction between aryl nitrile oxides and dienes, see Scheme 1. Various conditions for the generation of nitrile oxide from either the oxime or the chloroxime were investigated to determine the effect of reaction conditions on regioselectivity of the products. The effects of electronic turning of the aryl oxime on the selectivity of the [3+2] cycloaddtion reactions have been investigated and found to give the highest selectivity for electron-rich aryl groups. Unfortunately, the two products proved very difficult to separate. Therefore, additional experiments were performed to investigate how reaction conditions could improve selectivity. Only slight improvements on selectivity were achieved, albeit at the expense of the yield of the cyloaddition reaction, so the steric effect of substituted dienes were explored on selectivity. The result of these investigations is reported herein as well as attempts to reduce the isooxazoline cycloaddition products to the corresponding isooxazolidines. The isooxazolidines products would then be substrates for the rearrangement reactions to give the substituted pyrollidines. These rearrangement reactions have not yet been investigated.
Scheme 1. Cycloaddition of oxime and various dienes
I. Steric modifications to dienes for [2+3] cycloaddition reactions with phenyl nitrile oxide
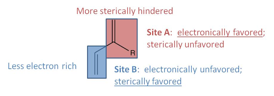
Our investigations on developing methodology for the highly selective synthesis of vinyl-substituted isooxazolines via a [2+3] cycloaddition reaction between nitrile oxide and diene suggest that only moderate fluctuations in product selectivities can be obtained via the reaction conditions or electronic tuning of the nitrile oxide. Therefore, we turned our attention to the steric and electronic tuning of the diene (see Figure 1). Our future efforts will focus on the synthesis of a variety of non-symmetrically substituted dienes to use as substrates in the cycloaddition reaction.
Figure 1. Electronic tuning hypothesis for diene
The use of myrcene as the diene instead of isoprene resulted in a single cycloadduct as the product. However, stability issues with the product has prohibited full characterization and yield determination.
II. Reduction of the isooxazoline to isooxazolidine
The reduction of the vinyl-substituted isooxazoline cycloaddition products to the corresponding isooxazolidines without cleavage of the N-O bond was expected to a challenging hurdle to overcome in the project. Various reduction conditions have been employed, but no desired products have been observed.
Figure 2. Proposed Reduction of Isooxazoline and Subsequent Rearrangement Reaction
III. Student impact
I have had the pleasure of working with 9 undergraduate students over the course of 2 years on this PRF grant. These students have been exposed to 1H-NMR interpretation, GC-MS analysis, HPLC method development, air-free reaction set-up techniques, monitoring reactions via TLC, and purification of organic products via silica gel chromatography. Exposure to the problem-solving approach inherent in any research endeavor will greatly benefit these young men and woman as they continue in their scientific careers. One of the students is now working for the State of Florida in the FAST fellowship program and plans to attend graduate school for chemistry in Fall 2018. Two others students are now second year medical students at Mercer University and the Medical College of Georgia. Another student completed a 6-month chemistry internship at Walt Disney World and is now a first-year graduate student at the University of Georgia. Three other student have been accepted into pharmacy schools. The other two students will graduate from Valdosta State University in Spring 2017 and plan to continue in scientific careers. This grant form the PRF has allowed these students a tremendous opportunity of being exposed to a research environment where they have honed their problem-solving and analytical reasoning skills.