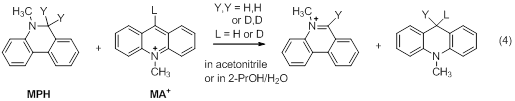Reports: UR455140-UR4: Structural Effects on the Primary Isotope Dependence of Secondary Kinetic Isotope Effects in Hydride Transfer Reactions in Solution
Yun Lu, Southern Illinois University (Edwardsville)
James E. Eilers, PhD, Southern Illinois University, Edwardsville
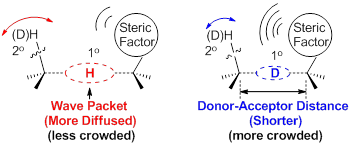
Figure 1. It is hypothesized that the steric factor will increase the 1o isotope effect (i.e. the DAD effect) on the 2o KIEs. The 2o H/D is at α- or β-position. The oval-shaped area is meant to show the wave-packet of the transferring H/D in the TRSs.
1. Steric effects on the primary isotope dependence of 2° KIEs in Hydride Transfer Reactions in Solution
This project investigates the hypothesis by determining the 1o isotope effect on the α- and β-2o KIEs for hydride transfer reactions from various hydride donors to different carbocationic hydride acceptors in acetonitrile or 2-propanol/water (1/4, v/v) (eqns (1) to (4), the counter ion for the carbocations: BF4-). The systems were designed to include the interactions of the steric groups and the targeted 2o H/D’s in the TRS’s so that the steric effects on the 2o KIEs can be differentiated for the 1o-H tunneling and D tunneling that have different DAD in their TRS’s.
Our findings include the following which support our hypothesis.
(1) Most 2o KIE values do not conform to the KIE values predicted from the semi-classical TS theory;
(2) The steric effect can augment the 1o isotope dependence of 2o KIEs, and the β-CH3/CD3 2o KIE is more sensitive to the 1o isotope effect than is the α-H/D 2o KIE;
(3) The 1o isotope dependence of 2o KIEs can imply information about the structure of the TRS’s;
(4) The significant 1o isotope dependence of α-2o KIEs reported by Kreevoy in 1983 for reaction (4) (and which has been frequently cited in the 1o isotope dependence of 2o KIE studies in biological H-transfer reactions) is not reproduced in our work.
2. Computational replication of the 1o isotope dependence of 2o kinetic isotope effects in solution hydride transfer reactions: Supporting the isotopically different TRS conformations
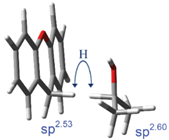
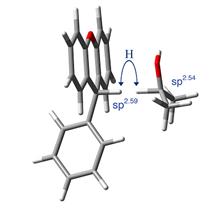
The above experimentally observed unusual magnitude of 2o KIEs at the β-2o CH3/CD3 position of 2-propanol in its hydride transfer to (T)Xn+ and Ph(T)Xn+ (see eqn (1)), and those at the 9-α-H/D position of the (T)Xn+, are replicated computationally following an activated motion-assisted H-tunneling model. All geometry optimizations, potential energy surface scans, and frequency calculations were done with Gaussian09 at the B3LYP/6-31+G(d) level. The frequencies were used to calculate the 2o KIEs using the ISOEFF07 program. The calculated effect of DAD (from 2.9 Å to 3.5 Å) on these 2o KIEs fits to the observed 1o isotope effect on 2o KIEs, suggesting that the 1o isotope effect is indeed a DAD effect and the H-tunneling has a longer DAD than the D-tunneling. This is consistent with our above qualitative explanation on the experimental results. The modeling of the 2o KIEs for both H- and D-transfers gives best-fit TRS structures which DAD for the sterically hindered system of Ph(T)Xn+ is interestingly not longer than that for the (T)Xn+ system (Figure 2). The DAD order predicts a smaller 1o KIE in the PhXn+ reaction than in the Xn+ reaction, which is contrary to the order of the observed 1o KIEs, suggesting a stronger DAD-compression vibrational mode on the reaction coordinate of the Ph(T)Xn+ system. The constructive vibrational modes have been proposed in the enzymatic systems as a possible reason to promote enzyme catalysis. This work for the first time demonstrates the existence of such vibration modes in the solution reactions as well.
|
Figure 2. The best-fit (average) TRS structures of the reactions of 2-propanol with Xn+ (left) and PhXn+ (right). The DADs are both 3.1 Å. Hybridizations of both donor and acceptor carbons are shown. Although the transferring hydride is shown in both the donor and acceptor positions, it is actually delocalized between the two.
Impact of the Research
This project allows to develop a new research direction in the PIs’ research groups. It also opens a new direction in the field of the physical organic chemistry that studies the structure-reactivity relationship for H-tunneling mechanisms. The published and preliminary results will lead the PI(s) to apply for the NSF or NIH grant that will expand the study on hydride transfer reactions and furthermore investigate the same for proton and hydrogen-atom transfer reactions.
Undergraduate and MS degree students involved in this project have learned kinetic procedures, basic organic synthesis, mechanistic analysis techniques, computational chemistry and the use of modern analytical instrumentation. The knowledge and techniques will make students competitive in their future careers. With these research experiences students are prepared to enter professional schools and Ph.D. programs, to become teachers, and problem-solvers for chemical companies. For example, a MS student partially supported by the grant is now in UC Davis for Ph.D. study, and an undergraduate student under the support currently studies pharmaceutical sciences in the SIUE pharmacy school.