Reports: DNI454396-DNI4: Probing Spin-Exciton and Spin-Charge Interactions in Open-Shell Organic Semiconductors
Trisha L. Andrew, University of Wisconsin-Madison
Research Summary
We answered two main questions about the structure-property relationships of spin-exciton interactions between spin _ organic radicals and organic chromophores.
1. What are the effects of through-space dipolar spin coupling on charge transport and excitonic properties and what are the spatial limits of this type of interaction?
2. What are the energy level limits for excitonic coupling between a radical SOMO and a semiconductor HOMO/LUMO?
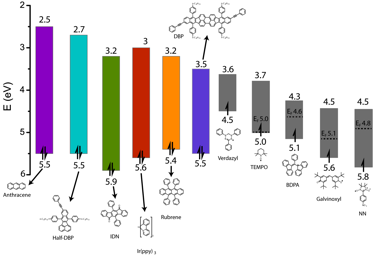
To answer these questions, first, a series of small-molecule radicals were synthesized and their electronic levels were characterized using ultraviolet photoelectron spectroscopy (UPS, for HOMO level measurements) and Kelvin probe force microscopy (KPFM, for work function measurements). Second, solution quenching studies (Stern-Volmer quenching experiments) were conducted using these small-molecule radicals as potential quenchers for a series of molecular organic chromophores of varying band gaps and HOMO/LUMO levels. The intent of this experiment was to establish energy boundaries for excited state quenching by a spin molecule and to, ideally, observe a correlation between the energy difference of the chromophore HOMO/LUMO and radical SOMO and quenching efficiency.
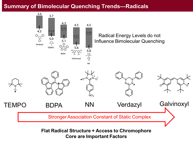
The results of this experiment were surprising, though all the radicals used in this study quenched the emission of the chromophores, no energy level correlation could be observed to explain the observed trend in quenching efficiency. This conclusion is starkly different than quenching by traditional, closed shell quenchers, in which case the energy offset between the chromophore and quencher LUMO levels exponentially affects quenching efficiency.
The results of this experiment revealed that a flat radical structure and spatial access to the chromophore core (needed to form a static association complex) are the main factors influencing bimolecular quenching efficiency.
This conclusion means that through-space radical-exciton interactions are very weak in magnitude (<10 meV). Further, these experiments revealed that, in order to observe meaningful radical exciton interactions in organic systems, through-bond coupling interactions need to be introduced into the molecular structure.
In the course of answering the
aforementioned two questions, a small series of regioregular, alternating
side-chain radical functionalized poly(thiophene)s were also synthesized, with
the intention of these polymers serving as controlled model systems to probe
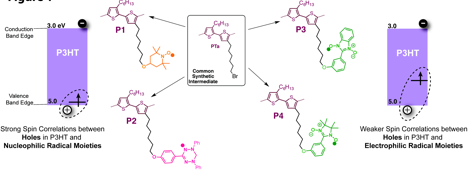
solid state interactions. Our modular approach used a common synthetic
intermediate, PTa, and a simple nucleophilic
substitution reaction to produce chemically pure derivatives via
post-polymerization functionalization. Polymer PTa was accessed via a robust GRIM polymerization reaction and was
therefore isolated in >95% yield as a regioregular, medium molecular-weight polymer
(20-30 KDa, depending on reaction batch). Polymers P1-P4 were all isolated
in 100-500 mg quantities as metallic-orange powders that are soluble in
chloroform, THF, toluene, dichlorobenzene and other nonpolar organic solvents.<
p>
We confirmed that post-polymerization functionalization of PTa did not change the molecular weight of the starting material using calibrated gel permeation chromatography. The presence of persistent radical moieties in P1-4 after post-polymerization functionalization of PTa was confirmed using CW EPR spectroscopy. Further, all radical materials were succesfully reacted with either ascorbic acid or phenylhydrazine to quantitatively quench the free radical moieties and produce diamagnetic compounds whose 1H and 13C NMR spectra were recorded to confirm structures and sample purity.
The observed oxidation onset potentials (aka valence band edges) and optical band gaps of P1, P3 and P4 were similar to those of PTa and P3HT in dilute solutions, confirming that the radical side-chain substituents are electronically isolated from the polymer backbone. We can therefore tentatively assume that the band edges of P1, P3 and P4 are equivalent to those of the unsubstituted parent polymer, P3HT.
Research Impact
Polymers P1-4 are the first known examples of medium molecular weight, side-chain radical substituted regioregular polythiophenes. We note that poly(3-arylthiophene)s containing pendant radical groups were synthesized over a decade earlier by Lahti and Nishide. However, since the GRIM polymerization method was unknown at the time, these previously-reported radical substituted polymers were either low molecular-weight (reported DP of 6) oligomers or regiorandom polymers that were possibly contaminated with paramagnetic iron ions. In comparison, the polymers synthesized herein are medium-to-high molecular weight (DP >100), regioregular, alternating poly(thiophene)s and the simple post-polymerization functionalization method that we employ to introduce radical moieties into the side chain is known to proceed in >98% yield. Therefore, synthetic strategy uncovered through this effort will be useful to produce soft magnetic materials for spintronic devices.
Impact on PI's Career
Dr. Jingjing Zhang, the postdoctoral researcher who worked on the synthesis of radical substituted poly(thiophene)s, succesfully obtained an entry position with Argonne National Labs and started his position in March 2016. One DARPA and one NSF proposal was developed based on the materials synthesized by the efforts described herein.