Reports: ND1054328-ND10: Novel Interrupted Polyacenes
Gordon W. Gribble, PhD, Dartmouth College
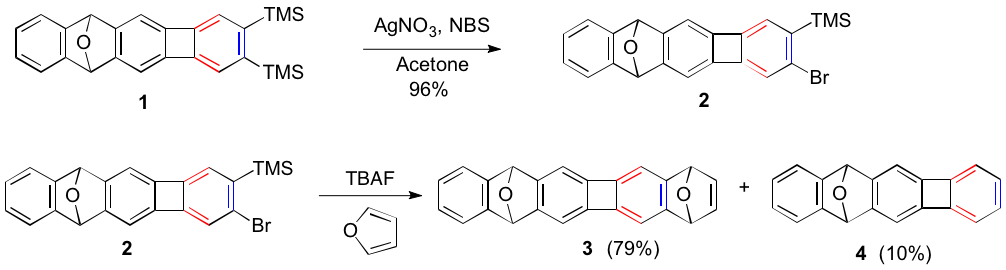
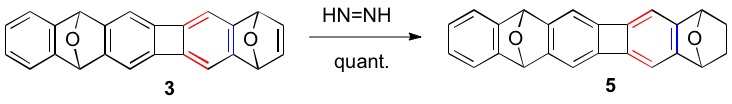
We have synthesized our first target polyacenes as their oxygen bridged analogues as shown below. Notably, we discovered that aryne generation can be accomplished from the appropriate o-bromo silyated arene as shown for the conversion of 1 to 2, and then 2 to a mixture of the desired 3 as the major product and 4 as a minor product. We are currently elaborating 3 to the polyacene 4. For example, diimide reduction of 3 gives 5 in quantitative yield and further stepwise dehydrogenation/deoxygenation should afford 4.
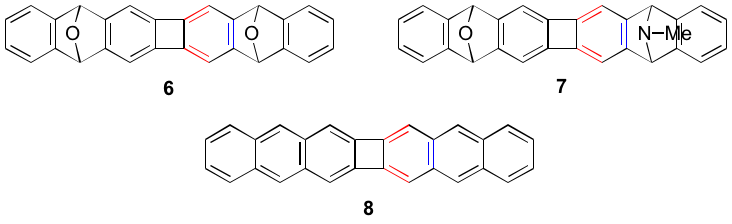
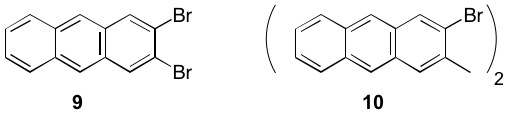
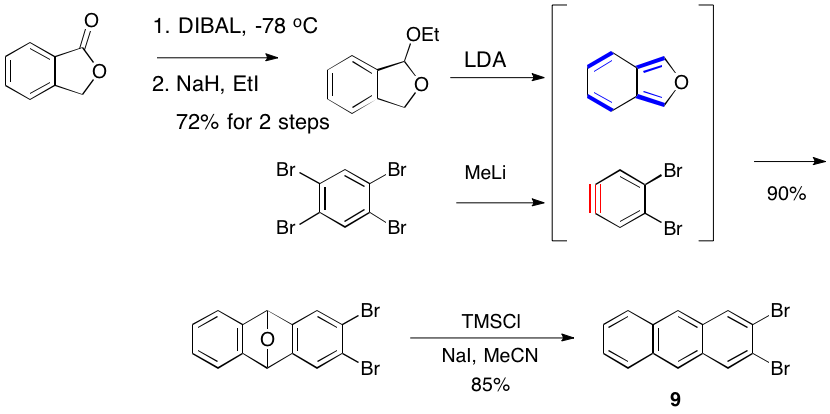
Likewise, we are examining the Diels-Alder reactions of 2 with isobenzofuran and N-methylisoindole, which should afford targets 6 and 7, respectively. The elaboration of 6 and will provide our interrupted bis-anthracene 8. Attempts to prepare 8 by dimerization of dibromoanthracene 9 and bis-bromoanthracene 10 have been not successful thus far. The novel 10 was prepared by mono lithiation of 9 followed by Pd-catalyzed coupling.
As shown below we have improved upon the synthesis of known 2,3-dibromoanthracene (9).
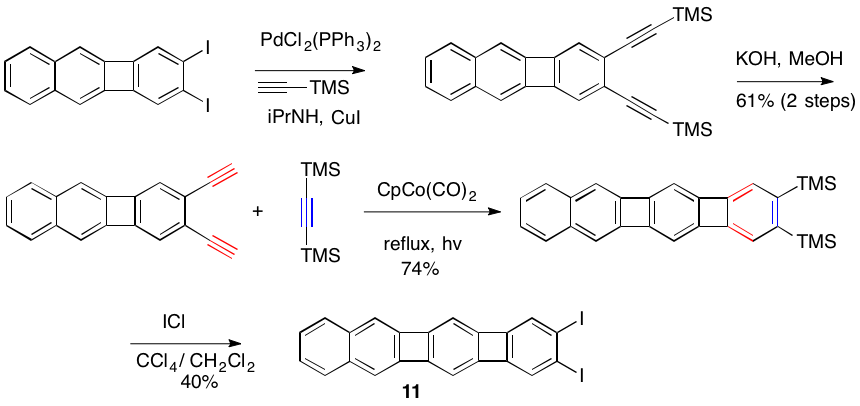
Our synthesis of the key bis-biphenylene compound 11 is shown below. This unit will be employed to prepare extended polyacenes.