Reports: DNI154422-DNI1: Cu-Catalyzed Vicinal Dicarbofunctionalization of Simple Alkenes
Michael Brown, Ph.D., Indiana University
Overview: This PRF grant for the last year has been used to support the development of an enantioselective interrupted cross-coupling reaction. This was a key objective that was outlined in the PRF proposal.
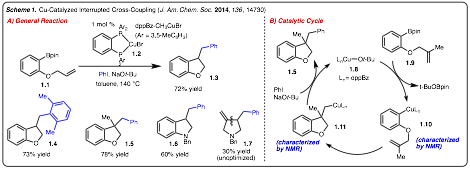
In 2014 we published our results detailing a Cu-catalyzed interrupted cross-coupling that lead to diarylation of alkenes (i.e., vicinal dicarbofunctionalization of alkenes). We have identified that treatment of readily prepared substrate 1.1 with Cu-catalyst 1.2, PhI and NaOt-Bu led to the formation of benzofuran in good yield (Scheme 2A). This process has been extended to various aryl iodides (e.g., 1.4) and to the formation of nitrogen heterocycles (e.g., 1.6-1.7). The catalytic cycle of this reaction has been determined by isolation of key intermediates and proceed by the following elementary steps: 1) transmetallation to generate Cu-complex 1.10, 2) migratory insertion of the pendant alkene into the Cu-Ar bond to furnish 1.11, and 3) capture of the Csp3-Cu-complex 1.11 with PhI (Scheme 2B).
Within the last funding period, the method depicted in Scheme 1, has been to extended to include enantioselective variants. This manuscript was published late 2015 in J. Am Chem. Soc. The details of this method are briefly outlined below. Through the evaluation of a set of chiral ligands related to dppBz, use of 3 mol % BenzP* 2.3 was effective for converting 2.1 to 2.4 in 55% yield and 97:3 er (Scheme 3). Furthermore, highly enantioselective synthesis of quaternary carbons is also possible (e.g., 1.2.5). The substrate scope has been found to include a variety of aryl iodides and tolerate the formation of indoline products. Some of the notable aspects of the study include: 1) Stereically hindered quaternary carbons can be generated (product 2.16). 2) Sterically hindered aryl iodides can be used (product 2.13). 3) Activated arylbromides were also found to be suitable substrates (product 2.8).
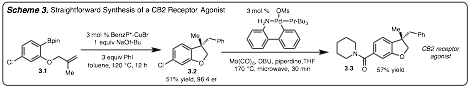
The utility of this method was demonstrated toward the synthesis of a CB2 receptor agonist (3.3). Synthesis of 3.3 commenced with reaction of 3.1 under standard conditions to produce 3.2 in good enantioselectivity. Palladium-catalyzed amino carbonylation of 3.2 led to the formation of 3.3 in 57% yield.
Current Directions: The continuation of this program has been directed towards two areas. The first is the extension of the results for diarylation with activated arylbromides. We are currently working on developing a general set of conditions that allow for Cu-catalyzed Suzuki-Miyarua type cross-coupling of arylbromides. Prior efforts from our lab (preliminary results for the PRF proposal) focused on developing a Cu-catalyzed Suzuki-Miyarua cross-coupling of aryl iodides. Extension of this process to coupling of aryl bromides is challenging but significant as aryl bromides are widely available (as compared to aryl iodides). The second research direction is the carboboration of allenes under Cu-catalysis. We have already identified that intramolecular coupling can be achieved to prepare indoline type products. Both of these programs aim to investigate fundamental reactivity of Cu-catalysis as applied to the conversion of simple hydrocarbon based compounds to more complex products.
Conclusions: Through development of the two processes outlined above, solutions to the long-standing challenge of vicinal dicarbofunctionalization of unactivated alkenes have been provided. The reactivity uncovered by the methods outlined above is leading to the development of other interesting avenues in Cu-catalyzed cross-coupling.