Reports: ND455631-ND4: Novel Electrostatically Activated Bronsted Acids
Steven Kass, University of Minnesota
Brønsted acids are among the most commonly employed catalysts and are used to promote a wide variety of chemical transformations. They provide an excellent alternative to transition metal containing catalysts in that they tend to be tolerant of water and air, and generally are environmentally friendlier. Substrate activation commonly takes place by proton transfer or hydrogen bonding, both of which lower the energy of the lowest unoccupied molecular orbital (LUMO) and facilitate nucleophilic attack. Different types of Brønsted acid catalysts with a wide range of acidities have been developed including thioureas, a,a,a,a-tetraaryl-1,3-dioxolane-4,5-dimethanols (TADDOLs), 1,1'-bi-2-naphthol (BINOL) derivatives and phosphoric acids, and their utility has been amply demonstrated. Of these compounds, phosphoric acids are inherently the strongest acids, and their structural flexibility enables them to be introduced into rigid organic frameworks. The presence of a phosphoryl oxygen also can serve as a hydrogen bond accepting site enabling dual or bifunctional activation modes that increase their catalytic potential.
Diphenyl phosphate [(PhO)2P(O)OH, DPP] is widely used and can catalyze a variety of transformations such as Friedel-Crafts and Diels-Alder reactions, and ring-opening polymerizations. Its reactivity is limited due to its modest acidity but can be enhanced by the presence of strong electron withdrawing groups on the two aromatic rings. More acidic derivatives are of interest especially since there is a relationship between catalyst acidity and activity (i.e., more acidic catalysts lead to faster rates.
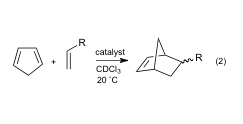
In this regard, electrostatically enhanced phosphoric acids 1 and 2were synthesized and their reactivity was explored in a number of reactions including a
Brønsted acids are among the most commonly employed catalysts and are used to promote a wide variety of chemical transformations. They provide an excellent alternative to transition metal containing catalysts in that they tend to be tolerant of water and air, and generally are environmentally friendlier. Substrate activation commonly takes place by proton transfer or hydrogen bonding, both of which lower the energy of the lowest unoccupied molecular orbital (LUMO) and facilitate nucleophilic attack. Different types of Brønsted acid catalysts with a wide range of acidities have been developed including thioureas, a,a,a,a-tetraaryl-1,3-dioxolane-4,5-dimethanols (TADDOLs), 1,1'-bi-2-naphthol (BINOL) derivatives and phosphoric acids, and their utility has been amply demonstrated. Of these compounds, phosphoric acids are inherently the strongest acids, and their structural flexibility enables them to be introduced into rigid organic frameworks. The presence of a phosphoryl oxygen also can serve as a hydrogen bond accepting site enabling dual or bifunctional activation modes that increase their catalytic potential.
Diphenyl phosphate [(PhO)2P(O)OH, DPP] is widely used and can catalyze a variety of transformations such as Friedel-Crafts and Diels-Alder reactions, and ring-opening polymerizations. Its reactivity is limited due to its modest acidity but can be enhanced by the presence of strong electron withdrawing groups on the two aromatic rings. More acidic derivatives are of interest especially since there is a relationship between catalyst acidity and activity (i.e., more acidic catalysts lead to faster rates.
In this regard, electrostatically enhanced phosphoric acids 1 and 2 were synthesized and their reactivity was explored in a number of reactions including a Friedel-Crafts alkylation (eq. 1) and a Diels-Alder cycloaddition (eq. 2). The rates of these reactions were measured under a variety of conditions and compared to DPP-catalyzed processes. Rate accelerations of up to 1,000 and 2,5000 were observed for 1 and 2, respectively. These compounds are the first examples of phosphoric acids with positively charged centers incorporated to enhance their acidities and catalytic abilities in nonpolar media.