Reports: DNI1053678-DNI10: New Strategies to Synthesize Covalent Organic Frameworks
Jian Zhang, University of Nebraska-Lincoln
The goal of this project was to develop new methods for the synthesis of covalent organic frameworks and porous organic frameworks. In the first year of this grant, we have developed a facile synthetic approach to access carbazolic porous organic frameworks (Cz-POFs) and further explored their visible light photoredox catalytic activities. In the second year, we developed two different approaches to prepare Cz-POFs with tunable photoredox properties and evaluated their applications in organic synthesis.
One versatile approach to fine-tune the optoelectronic properties of Cz-POFs, which exhibit mostly donor properties, is to incorporate electron acceptor (A) moieties into the polycarbazole framework and generate D-A Cz-POF. Forming D-A pairs is a common strategy to modify HOMO-LUMO levels for linear conjugated molecules/polymers for applications in light-emitting diodes, sensors, and photovoltaics. Currently, the highly complex intra-/intermolecular energy and electron transfer processes associated with D-A conjugated polymers, including emerging concepts of excess energy induced charge photo-generation and charge-transfer induced singlet fission, are under vigorous investigation, and much less is known about their photoredox properties and catalytic implementation.
1. Cz-POFs constructed by donor-bridge-acceptor (D-B-A) monomers
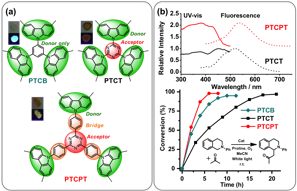
Inserting a bridge moiety (B) between D and A is expected to decrease their direct electronic coupling and weaken the charge transfer interaction. The size, electronic structure, composition, and bond saturation of the bridge all play an important role in governing not only the HOMO-LUMO gap, but also the exciton's generation and migration. Our study (Fig. 1) reveals that when a phenyl group was inserted between the D-A pair in polymer PTCPT (D = carbazole, A = triazine), we observed a significant change of optical and electronic properties compared to its prototypic polymer PTCT (D-A), likely due to a decreased CT interaction (Fig. 1). What is more intriguing is that, despite of its weaker photoredox potentials (E1/2P*/P_ = +1.06 V and E1/2P+/P* = –1.10 V), PTCPT (D-B-A) exhibited a faster conversion rate toward the Mannich reaction (Fig. 1b) compared to PTCT (D-A, E1/2P*/P_ = +1.55 V) and PTCB (donor only, E1/2P+/P* = –1.73 V) (Fig. 1b). This result does strongly suggest the importance of other photophysical factors besides redox potentials and the necessity of a systematic investigation.
Fig. 1. (a) Structure of Cz-POF PTCB (donor only), PTCT (D-A), and PTCPT (D-B-A). (b) UV-vis and fluorescence spectra of PTCT and PTCPT, as well as their rates of conversion toward Mannich reaction.
2. New Donor-Acceptor Monomer for the Construction D-A Cz-POFs
With the support of ACS-PRF, we recently stated a project aims to identify suitable monomers for construction of D-A Cz-POFs that can undergo proton-couple electron transfer (PCET) process. The motivations behind this studies is that PCET is an efficient way to modulate the ET kinetics and thermodynamics, which involves the concomitant transfer of a proton and an electron to an organic substrate in a single concerted process.
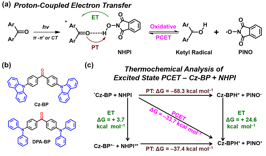
We choose ketone is a weak H-bond acceptor since the presence of an electron donor such as amine in ketone-based D-A fluorophores would significantly enhance their interaction with a suitable H-bond donor due to the enhanced basicity at both ground and excited state. Here, benzophenone (BP) is chosen as the structural prototype since the para-position of the phenyl groups in BP can be substituted with an electron donor to afford D-A fluorophores. In the presence of H-bond donor NHPI, the photo-excited ketone can undergo the PCET to form the ketyl and PINO radicals (Fig. 2a). We choose two donor-substituted BP that exhibit the lowest CT, namely, Cz-BP (4,4'-bis(9-carbazolyl)-benzophenone) and DPA-BP (4,4'-bis(diphenylamino)-benzophenone) (Fig. 2b) and performed a simplified thermochemical analysis for Cz-BP. Supportive thermodynamic parameters for the hydrogen transfer from NHPI (Fig. 10c) were obtained: electron transfer in both stepwise ET-PT (_GETo = +3.7 kcal mol-1) and PT-ET (_GETo = +24.6 kcal mol-1) processes would likely encounter high-energy intermediates; in contrast, the concerted PCET step (_GPCETo = –33.7 kcal mol-1) exhibits significantly more favorable thermochemical energetics as well as fast kinetics to generate PINO radicals.
Fig. 2. (a) Scheme of PCET from NHPI to an aromatic ketone. (b) Structures of DMA-BP, Cz-BP, and DPA-BP. (c) Thermochemical analysis of excited state PCET for Cz-BP and NHPI.
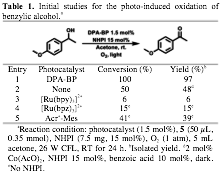
We choose benzyl alcohol oxidation to evaluate the efficiencies of the photochemical generation of PINO radicals using our designed D-A fluorophores (Table 1). After irradiation of the reaction mixture with a 26 W CFL under 1 atm O2 for 24 h in acetone, the ketone product was obtained in an excellent yield (97%, Table 1, entry 1). Light irradiation, a photocatalyst, NHPI, and molecular oxygen are all essential to this reaction. Our catalytic system compares favorably to the metal-based Co(AcO)2/NHPI/O2 catalytic system under the same reaction condition (48% yield, Table 1, entry 2). The synergistic catalytic activity of photocatalyst and PINO is essential for this reaction. For instance, moderately oxidative photocatalyst such as [Ru(bpy)3]2+, which does not have H-bond interaction with NHPI, exhibited a diminished activity (6% yield, entry 3). In the absence of NHPI, despite their higher reduction potentials (EM+/M > +1.80 V) than that of the benzylic alcohol (E+/0 > +1.67 V), [Ru(bpz)3]2+ and Acr-Mes+ resulted in a poor yield of the ketone product (<40% yield, Table 1, entry 4-5).
Summary.
Funding for this project from the past two years has allowed us to develop new synthetic strategies for functional porous organic frameworks and explore their novel applications in photoredox catalysis for organic transformations. The newly developed D-A Cz-POFs based heterogeneous solid exhibited enhanced photocatalytic activities. The recently study of D-A monomers has resulted in the identification of an efficient building block to Cz-POFs that have potential to be developed as photocatalysts for PCET processes to generate synthetically useful radicals. The PRF fund has allowed my research group to recently secure an NSF CAREER grant.