Reports: DNI755563-DNI7: A Radical Approach to Conjugated Polymers
Emily Pentzer, PhD, Case Western Reserve University
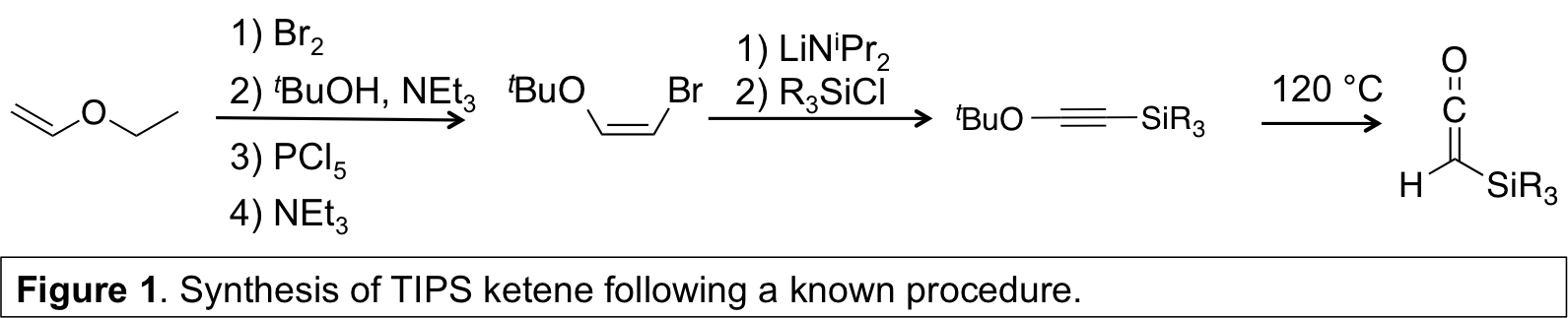
PI Pentzer has explored the polymerization of silyl ketenes under radical and anionic conditions. The monomer, triisopropyl silyl ketene (TIPS ketene) was synthesized by a reported method in overall good yield and purified by distillation (Figure 1). Initially, radical polymerization was explored and oligomerization of TIPS ketene was observed with oxygen-based radicals, including TEMPO and the radical derived from tBu2O2. Unfortunately, thorough evaluation of the reaction revealed that these oxygen-based radicals underwent reaction to yield alcohols (e.g., tBuOH, as previously reported), and that radical and nucleophilic mechanisms competed. As formation of alcohols could not be excluded, attention now focuses using nucleophiles as initiators.
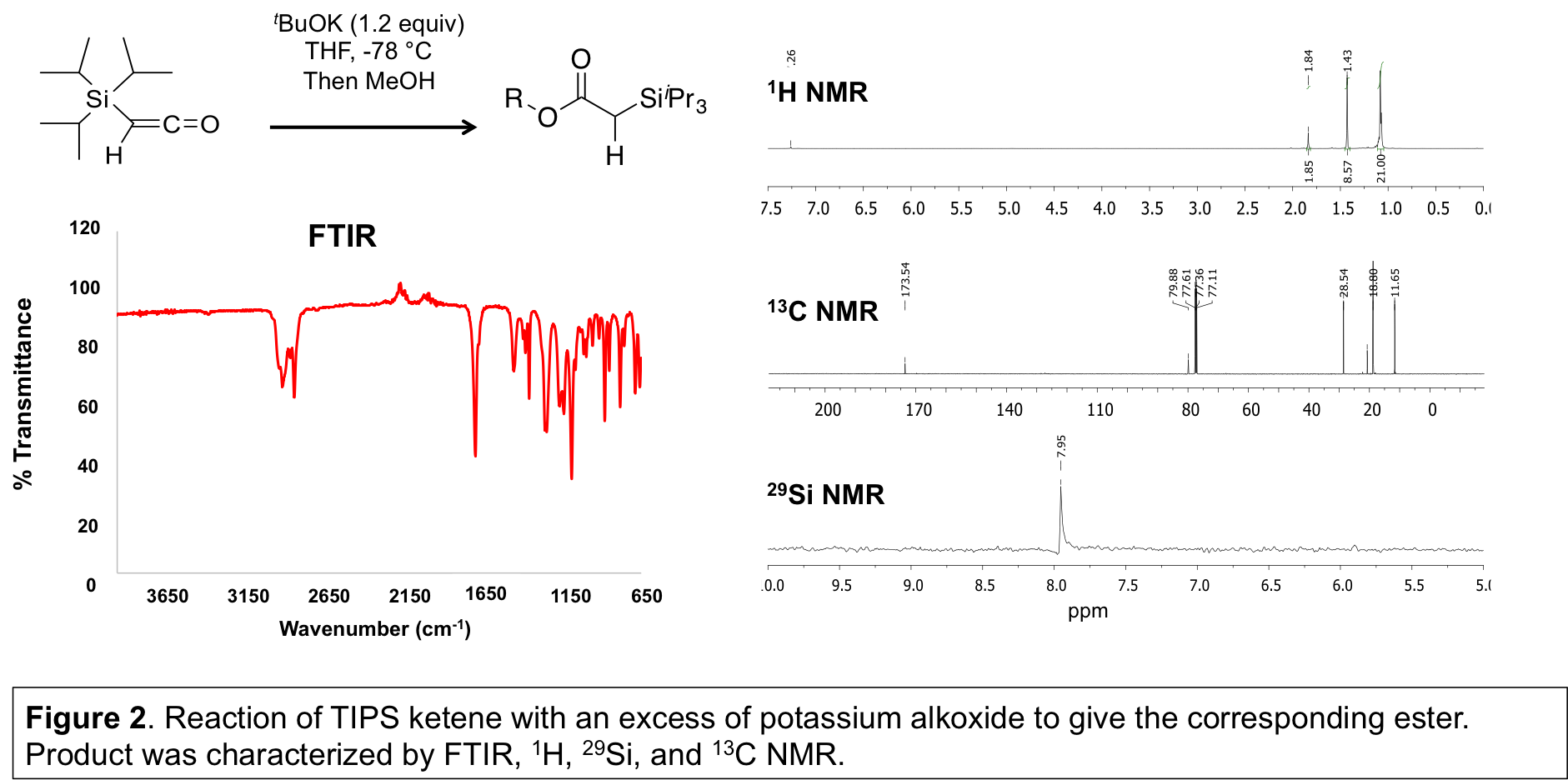
TIPS ketene was observed to react cleanly and efficiently with tBuOH in the presence of BF3, or KOtBu to give the ester enolate then corresponding ester (Figure 2). The product was characterized by FTIR, 1H, 13C, and 29Si NMR, GCMS, results not shown. This indicated that initiation (addition of alkoxide to silyl ketene) readily occurs. If the intermediate enolate can react with another equivalent of TIPS ketene, oligomerization will occur. Enolates are ambident nucleophiles and can react through either the carbon or oxygen atom, with selectivity dependent on substituents, counterion, solvent, temperature, and electrophile identity. This opens a pathway to form different polymers from one monomer, by propagation of the carbon to give a polyketone or propagation of the oxygen to give a polyketene acetal.
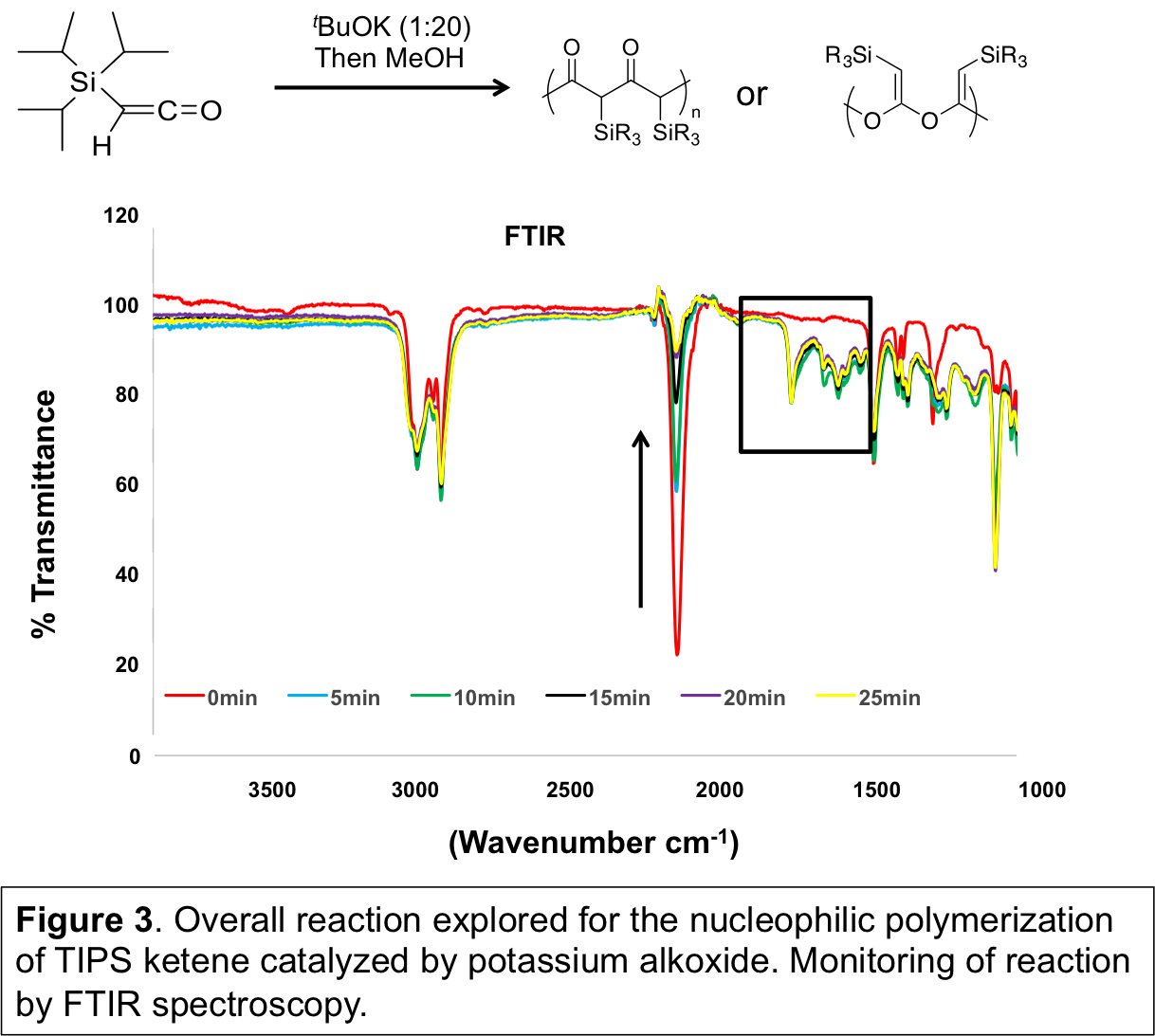
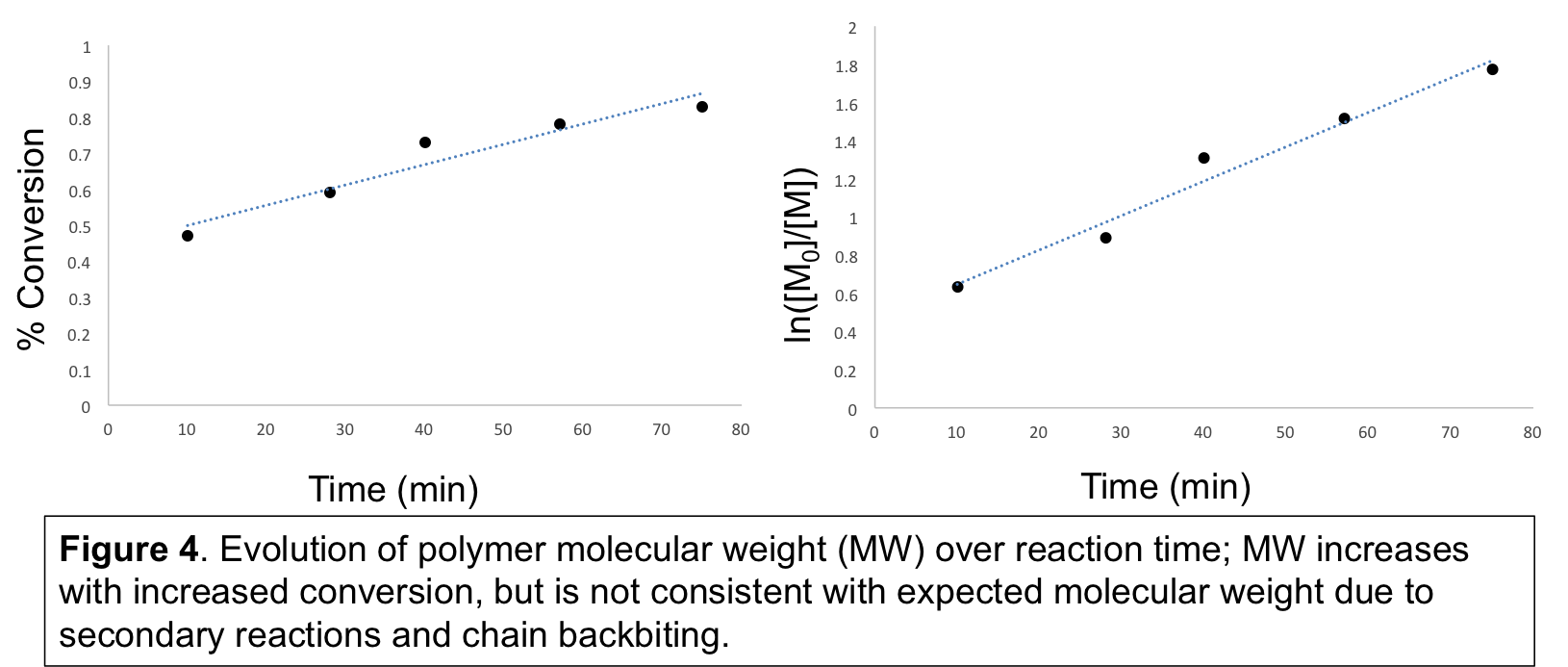
To observe if propagation occurred, an excess of TIPS ketene was used, and the reaction can be monitored by FTIR spectroscopy and disappearance of the signature ketene stretching frequency at ~2100 cm-1. In addition to disappearance of the TIPS ketene stretching frequency, peaks from ~1600-1750 cm-1 became apparent, indicative of carbonyl-containing species (Figure 3). Polymer formation was supported by gel permeation chromatography with detection by multi-angle light scattering (GPC-MALS), and increased consumption of TIPS ketene led to an increase in the molecular weight of the product (Figure 4). From 1H, 13C, and 29Si NMR, a complicated chemical structure was observed, indicated by the presence of ketene acetal, ketone, and ester functional groups, as well as enol forms of the ester and ketone. Secondary reactions, such as backbiting and chain transfer reactions, likely occurred as well, since the expected MW was lower than the observed MW, and MW decreased upon prolonged reaction time (data not shown).
The reaction of KOtBu with an excess of TIPS ketene led to propagation and formation of oligomers and polymers, thus indicating that the ester enolate derived from TIPS ketene is a nucleophile for a TIPS ketene electrophile! These exciting initial results indicate that silyl ketenes can undergo polymerization in the presence of an alkoxide initiator and that both C and O atoms propagate. Current work focuses on establishing a thorough understanding of the propagation of this polymerization in order to identity conditions for controlled product formation, and elimination of secondary reactions.