Reports: DNI152782-DNI1: Direct Access of Chemically Differentiated 1,2-Diols from Oxime-Masked Mono-Alcohols
Guangbin Dong, PhD, University of Texas at Austin
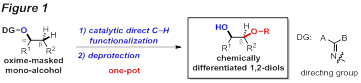
[Scientific Goals]: Our objective during the funding period is to develop a Pd-catalyzed C-CH functionalization strategy towards vicinal functionalization of mono-alcohols by using oximes as a removable directing group (Figure 1).
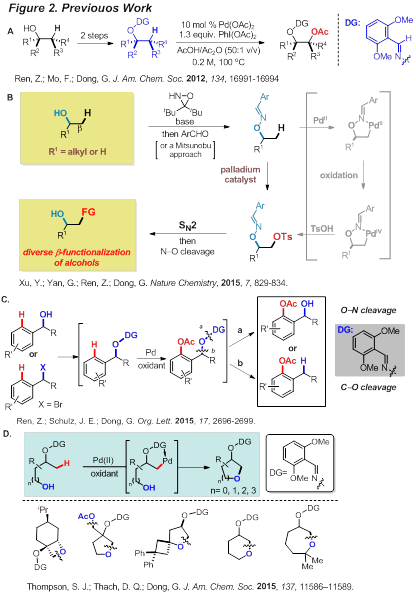
[Progress]: Previously, we developed a Pd-catalyzed vicinal oxidation of unactivated C-CH bonds (non-allylic/aromatic) using alcohol-derived oximes as directing groups (DG). As illustrated in Figure 2A, the oxime was employed as both a DG and an alcohol surrogate for this transformation. To enable diverse functionalization of an sp3 position that lacks pre-activation, we sought a new strategy that can directly replace a C-H bond with a good leaving group in a late-stage intermediate, and subsequently employ SN2 reactions, as a single type of transformation, for preparing derivatives or analogues (Figure 2B). To be specific, a sulfonyloxyl group can be installed at a β-C-H bond of a masked alcohol and subsequently SN2 reactions can be used to prepare various derivatives. On the other hand, a Pd-catalyzed ortho-acetoxylation of masked benzyl alcohols to synthesize various 2-hydroxyalkylphenol derivatives has also been developed (Figure 2C). Moreover, a palladium-catalyzed functionalization of unactivated sp3 C-H bonds with internal alcohol nucleophiles is also developed (Figure 2D).
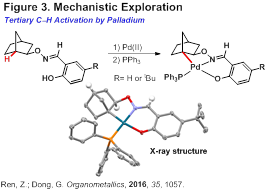 During the extended third year, significant progress has been made towards the above goal. The first focus was on exploration of the C-H metallation mechanism, particularly with the activation of tertiary carbons. Recently, we reported the first preparation of tertiary alkyl palladium complexes via C-H activation (Figure 3). Enabled by an exo-type oxime DG, cyclopalladation occurred smoothly at the bridgehead position. Treatment of the resulting complex with iodine led to C-CH iodination at the methine carbon. This study provides important mechanistic information for palladium-catalyzed functionalization of methine C¨CH bonds.
During the extended third year, significant progress has been made towards the above goal. The first focus was on exploration of the C-H metallation mechanism, particularly with the activation of tertiary carbons. Recently, we reported the first preparation of tertiary alkyl palladium complexes via C-H activation (Figure 3). Enabled by an exo-type oxime DG, cyclopalladation occurred smoothly at the bridgehead position. Treatment of the resulting complex with iodine led to C-CH iodination at the methine carbon. This study provides important mechanistic information for palladium-catalyzed functionalization of methine C¨CH bonds.
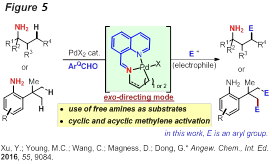
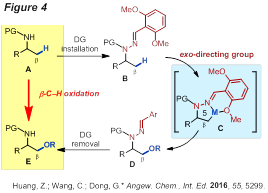 On the other hand, using a similar strategy, a new hydrazone-based exo-DG strategy was developed for the functionalization of unactivated primary β-C-CH bonds of aliphatic amines (Figure 4). Furthermore, to enable C-CH activation at the β position of the amines, the strategy of using an in situ generated imine-type DG was developed (Figure 5). One main merit of this strategy is allowing use of free unprotected primary amines as substrates. Methyl, cyclic and acyclic methylene groups can be activated. Importantly, the feasibility of catalytically using the DG component is also demonstrated.
On the other hand, using a similar strategy, a new hydrazone-based exo-DG strategy was developed for the functionalization of unactivated primary β-C-CH bonds of aliphatic amines (Figure 4). Furthermore, to enable C-CH activation at the β position of the amines, the strategy of using an in situ generated imine-type DG was developed (Figure 5). One main merit of this strategy is allowing use of free unprotected primary amines as substrates. Methyl, cyclic and acyclic methylene groups can be activated. Importantly, the feasibility of catalytically using the DG component is also demonstrated.
[Significance and Impact]: Our progress has made a significant impact: (1) a better understanding of the key C-H palladation has been obtained; (2) the strategy has been extended to C-H functionalization of amines; and (3) catalytic use of DGs has also been demonstrated. This exo-type DG strategy is expected to be widely employed in aliphatic C-H functionalization reactions. Two students on this project have successfully graduated with PhD degrees in Chemistry: one found a postdoc position at Emory University; the other started a position at RTI International.