Reports: UR152858-UR1: Effective, Catalyzed and Electrophilic Cyclization Reactions Leading to Highly and Diversely Substituted Fluoroheterocycles
Roman Dembinski, Oakland University
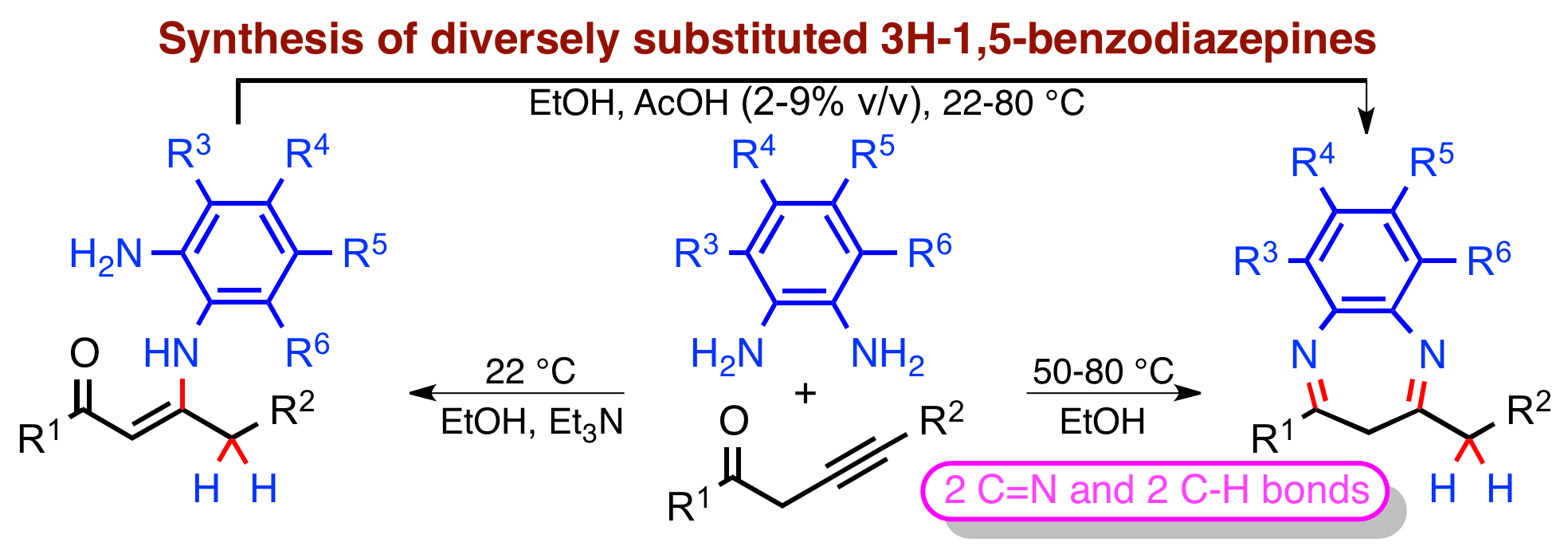
The reaction of alk-3-yn-1-ones (a bifunctional reagent) with o-phenylenediamines provides an effective synthetic method with high atom economy for the preparation of diversely substituted 1,5-3H-benzodiazepines. The reaction initially leads to the formation of conjugated enaminones (3-amino-2-alkenones, 51-99%), at room temperature, which constitutes a formal non-catalyzed hydroamination of the non-conjugated alkyne. Non-symmetrical o-phenylenediamines react in a regioselective fashion with respect to an amino group. Both the direct microwave-accelerated reaction of o-phenylenediamines with alkynones in ethanol and the intramolecular cyclization of intermediate enaminones in ethanol/acetic acid lead to substituted 1,5-benzodiazepines with good yields (39-92 and 26-99%). The regio- and stereochemical outcomes of these processes are confirmed by the X-ray structure determination of (Z)-3-[(2-amino-4-methylanilino)-4-(4-methylphenyl)-1-phenylbut-2-en-1-one, 4-[(4-methylphenyl)methyl]-7-nitro-2-phenyl-3H-1,5-benzodiazepine and 6,8-dimethyl-4-(4-methylbenzyl)-2-phenyl-3H-benzo[b][1,5]diazepine. Elaboration of this methodology opens access to 3-fluorobenzodiazepines, if 2-fluoroalk-3-yn-1-ones would be used.