Reports: UR454658-UR4: Interaction of Aromatic Hydrocarbons with a Cubic Self-Assembled Metallocage
John D. Thoburn, PhD, Randolph-Macon College
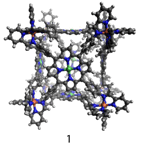
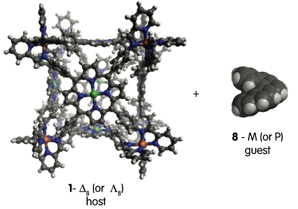
Many biological systems use non-covalent interactions to direct the self-assembly of subunits into larger arrays having highly specific functions. Chemists uses similar principles to design synthetic supramolecular assemblies that can fulfill a wide variety of applications. A recently developed supramolecular cube (1) built from 8 Fe2+ and 6 square planar porphyrins has shown promise for encapsulation of large aromatic guests, which could potentially be used to separate polycyclic aromatic petroleum products such as vandal porphyrins from asphaltene.
Our goal is to study the underlying science of how these M8L6 porphyrin-based metallocomplexes self-assemble, how they encapsulate guests, and how they can be synthetically modify them to make them more soluble and thereby improve their utility. Thus far we have focused on synthesis and have made steady progress on the synthesis of derivatives of host 1 as well as some potential guests for use in a dynamics and molecular recognition studies.
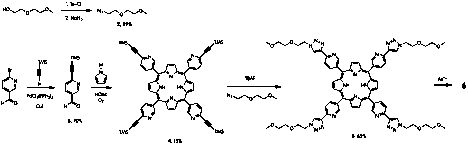
The original cubic metallocomplex 1 suffers from low solubility in virtually all solvents. In order to make these cubes more useful we are synthesizing metallocubes with pendant oligoethylene glycol (OEG) chains (6). The most direct approach to incorporating the solubilizing ethylene glycol chains into the metallocube involves the use of 'click' chemistry, i.e. coupling an ethylene glycol azide (2) with an acetylenated pyridyl porphyrin (4) as shown in Scheme 1.
Scheme 1. Synthetic scheme for preparation of cubic organic metallocomplex with pendant ethylene glycol chains.
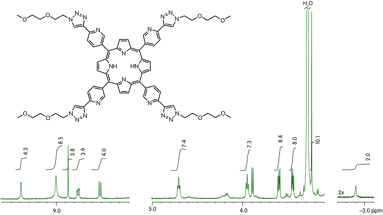
A classic Sonogashira coupling affords the appropriate TMS-acetylene benzaldehyde in high yields. Coupling with pyrrole yields the porphyrin precursor, which then undergoes a tandem deprotection and copper(I) catalyzed Huisgen cycloaddition. The 1H NMR of compound 5 (Figure 1) shows we have successfully synthesized the ligand and we are now ready to begin the self-assembly of the metallocubes with increased solubility.
Figure 1. 1H NMR of OEG tetra(triazolylpyridyl)porphyrin 5.
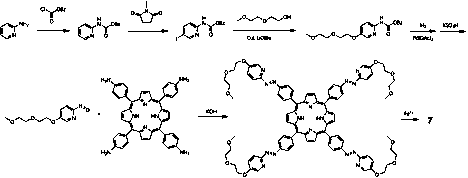
Concurrently, we are also working on an alternative ligand for binding the iron(II) anchoring the vertices of the cube. The original metallocube 1 is made by subcomponent self-assembly of an amine and an aldehyde to form an imine (ÐC=NÐ, Figure 2), which can chelate Fe(II). We are working on replacing the imine with an azo group (ÐN=NÐ, Figure 2), which can also chelate Fe(II), but only in the trans form. Under the influence of light the azo group can photoisomerize to the cis form, which can no longer chelate the iron (Figure 2). This will allow us to open the metallocage under photolytic conditions, as well as re-close with a different wavelength of light. In doing so we can control the rate at which guests enter and leave the host cage.
Figure 2. Imine chemistry of original host 1 compared to azo chemistry of modified cage 7 capable of cage opening under photolytic conditions.
Scheme 2. Synthesis of an azo variant of the metallocube via tetra(aminophenyl) porphyrin and a nitrosopyridine.
We have accomplished the first two steps of this synthesis as shown in Scheme 2 and will continue to pursue this avenue in the coming year.
Finally, we are investigating the interaction between the cubic host and a variety of guests. We wish to take advantage of the inherent handedness of these chiral metallocages to probe the chiral recognition properties with chiral guests. All eight metal centers of the cage have the same handedness (all Delta or all Lambda). Since it has already been established that large polycyclic aromatic molecules like coronene have a high affinity for these metallocomplexes, pentahelicene 8 appears to be an ideal chiral guest for this chiral host (Figure 3).
Figure 3. Helical guest pentahelicene 8 and chiral metallocomplex 1.
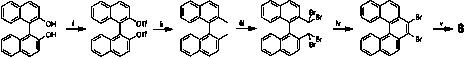
We have synthesized the necessary helicene albeit in very small quantities according to Scheme 3. While the first four steps of the synthesis have all gone in good yield (grams), the last step, which involves treatment with potassium t-butoxide, has produced only very small amounts of pentahelicene.
Scheme 3. Pentahelicene Synthesis.
We should be able to remove the bromines in higher yields by using n-butyl lithium and quenching with water. When the helicene 8 is synthesized we will look to see if 8-M, or its enantiomer 8-P, binds better to 1-Delta8 or 1-Lambda8.
This past year three undergraduate researchers have worked on these projects and their role in this endeavor has been essential. The success of the projects rest in their hands, which in turn is dependent on the training they receive from the PI. The challenges they have overcome in the laboratory are an important component of their education, their preparation for graduate school, and their careers. With support from the ACS-PRF one of the students has benefitted from the opportunity to present this work at the 251st national meeting of the American Chemical Society. We are very grateful to the ACS Petroleum Research Fund for making this project possible.