Reports: UR555886-UR5: In Situ Generated Two Dimensional Metal and Bimetallic Nanoparticle Catalysts
Krisanu Bandyopadhyay, University of Michigan (Dearborn)
Project Objective:
The objective of the present research is to generate nanoparticle (NP) assemblies in situ inside a suitable template on a solid surface to avoid a number of sequential steps and pre-synthesized nanoparticle in solution. Moreover, the template will essentially act as a reaction chamber that provides a scaffold for immobilization of specific metal ions, prevent aggregation, and further act as capping agent to control the growth of the desired nanoparticle structure. In this present research project, we propose to generate in situ 2D assemblies of metallic (Au), and bimetallic (Au-Pd) nanoparticles inside different silane monolayer templates on silicon and indium tin oxide (ITO)-coated glass surfaces. The present method is primarily based on self-assembly and takes advantage of the variation in solution pH to control the degree of ionization of the surface functional groups, which modulate the electrostatic interaction between the ions in solution and the immobilizing surface. The main focus of the present study is to control the size of the nanoparticles and study the size-dependent electrocatalytic property of these surface bound Au-Pd nanoparticle nanoparticles to investigate oxidation of higher molecular weight alcohols and polyalcohols that has direct significance for direct alcohol fuel cells (DAFCs).
A. Generate two dimensional assemblies of bimetallic Au-Pd nanoparticles:
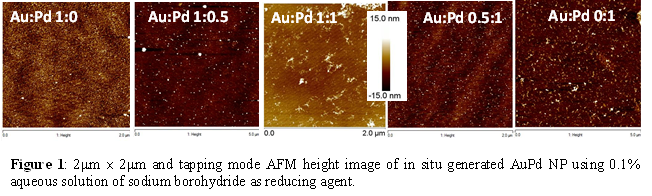
In our present study, we have generated bimetallic AuPd NPs varying Au:Pd mole ratio of 1:0, 1:0.5, 1:1, 0.5:1, and 0:1. Electrocatalytic activities of these systems have been evaluated for higher molecular weight and polyalcohol oxidation in alkaline medium. Bimetallic AuPd NPs assemblies are generated by simultaneous in situ reduction of [PdCl4]2- with [AuCl4]- ions bound to the TSPEI functionalized surface. The multiple amine functionalities present at the polyethyleneimine (PEI) backbone of TSPEI can entrap both [AuCl4]- and [PdCl4]2-) ions from solution through electrostatic interaction at a lower pH. Again in this synthesis, silicon and ITO have been used. The surface functionalization with TSPEI has achieved followed by AuPd bimetallic NPs generation using 1 ´ 10-2 M HAuCl4 and 1 ´ 10-2 M K2PdCl4 solutions which were made separately and mixed in different volumes to create the desired Au: Pd mole ratio in the final solution. After incubating the surfaces for 8 hours followed by rinsing with water and drying under a flow of argon, the surfaces with adsorbed ions were placed in a freshly prepared 0.1% (w/v) aqueous solution of sodium borohydride for 8 hours to generate the AuPd bimetallic NPs. Representative AFM images shows nearly uniform AuPd NPs on silicon surface (Figure 1) after the final reduction step with the different Au:Pd mole ratio in solution. In this experiment, K2PdCl4 is used as Pd source. An apparent change in the morphology of the nanoparticles is observed with increasing the concentration of palladium ions, the nanoparticles increase in size and number. The average nanoparticle size for Au:Pd 0:1 was ~7.40 nm, for Au:Pd 1:1, the average nanoparticle size was between ~2-3 nm and for Au:Pd 1:0.5 the average nanoparticle size was between ~0.8-1.4 nm as obtained from analysis of a number of AFM images of different samples.
B. Electrocatalytic behavior of bimetallic AuPd nanoparticles for higher molecular weight alcohols and polyalcohols:
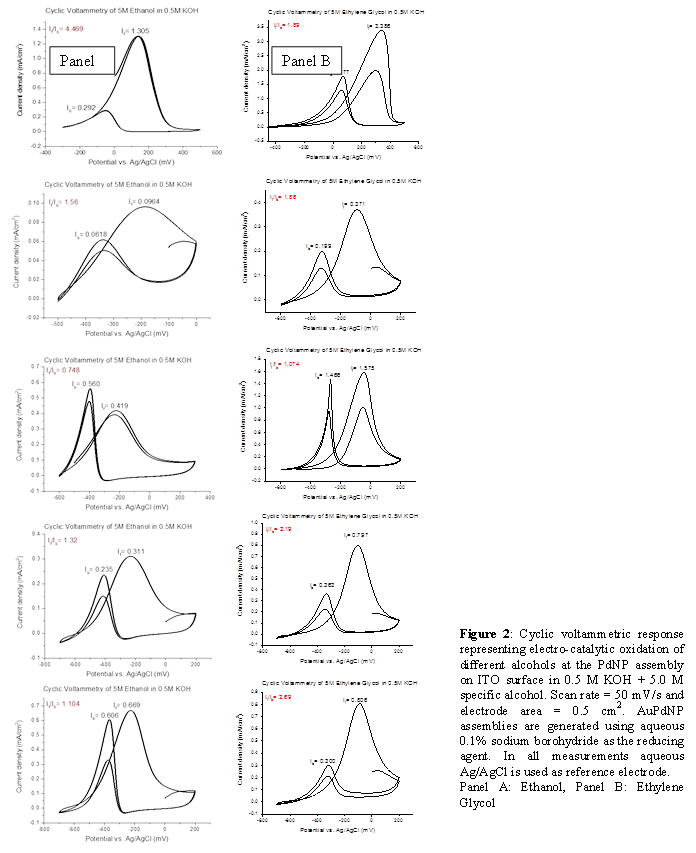
Preliminary results on electrocatalytic activity of PdNPs generated by borohydride reduction towards different alcohols are presented in Figure 2. A strong symmetric anodic oxidation peak during forward scan is the most noticeable feature in all of the electrocatalytic responses. In addition, all the votammograms show another anodic peak during reverse scan. This anodic peak in the reverse scan is attributed to the removal of the incompletely oxidized carbonaceous species formed in the forward scan. It is also known that accumulation of intermediate carbonaceous material on the catalyst surface leads to catalyst poisoning. Hence the ratio (If/Ib) of the forward anodic peak current density (If) to the reverse anodic peak current density (Ib), can be used to describe the catalyst tolerance of carbonaceous species accumulation. Low If/Ib ratio indicates poor oxidation of specific alcohol during anodic scan and excessive accumulation of carbonaceous residues on catalytic surface. High If/Ib ratio shows greater efficiency of the catalyst. Based on the If/Ib ratios, it is very obvious (Figure 2) that AuPdNP catalyst generated by borohydride reduction has shown that the AuPd nanoparticle assemblies are more efficient catalyst for ethanol with increase concentration of Au and a reverse effect was observed in case of ethylene glycol.
Future Experiments:
Currently X-ray photoelectron studies are in progress and will be correlated to the catalytic behavior of AuPd nanoparticles assemblies with varying ration of Au:Pd. In addition, in situ generation of anisotropic metallic and bimetallic structure also on-going.
Student participation, Publications and Presentations:
A total 7 undergraduate students have worked on this project so far. Among these, four students have graduated and two are in chemistry graduate program, one in medical program and one in nutrition program. A manuscript is in preparation dealing with AFM, electrochemistry and zeta potential results. The PI has also presented a part of the current work at the Spring ACS meeting at San Diego.