Reports: UNI155295-UNI1: Design, Synthesis, and Properties of Shape-Persistent Molecular Systems Comprising 1,3,5-Triethynylbenzene Cores
Derik K. Frantz, PhD, California Polytechnic State University
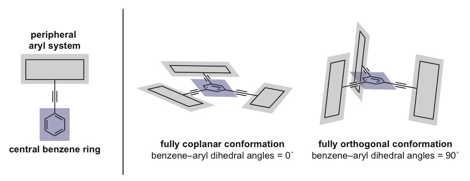
Over the past decade, arylethynylbenzenes have been applied toward the generation of carbon-rich molecules, liquid crystals, molecular knots, and materials with unusual optical and photophysical properties. Our efforts aim to explore the utility of the 1,3,5-triethynylbenzene moiety as a template for generating complex, shape-persistent structures in which the peripheral aryl systems are locked in coplanar and orthogonal conformations.
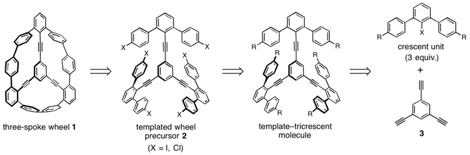
Our efforts toward a shape-persistent coplanar molecule have focused on
three-spoke wheel 1, which could serve as a precursor for novel
cyclophenylene-based structures. Using our triethynylbenzene template approach,
retrosynthetic analysis of 1 disconnects to halogenated precursor 2
that could generate 1 by intramolecular Ullmann coupling. Precursor 2
may be prepared by Sandmeyer-type chemistry from a template–tricrescent
molecule prepared by Sonogashira coupling of three crescent units to
1,3,5-triethynylbenzene (3).
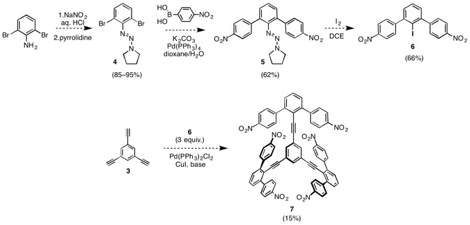
Our initial synthetic approach involved the use nitro groups that could be converted to iodides by late-stage Sandmeyer-type reactions. Commercially available 2,6-dibromoaniline is efficiently converted to triazene 4, which undergoes a two-fold Suzuki cross-coupling with 4-nitrobenzeneboronic acid to afford terphenytriazene 5. Treatment with I2 converts 5 to iodide 6, which undergoes three-fold Sonogashira coupling with 3 to afford hexanitro tricrescent compound 7. Efforts to increase the yield of the Sonogashira step are underway.
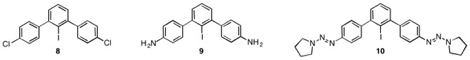
Current work focuses on completing the synthesis of 1 from 7 and developing alternative crescent units for use in the synthesis of 1. Dichloro-derivative 8 could eliminate the need for late-stage Sandmeyer chemistry by generating a hexachloro-derivative of 2 directly via Sonogashira coupling with 3. Several recent reports have demonstrated successful Pd-nanopartical-based homocoupling of aryl chlorides. Other targets include diamine 9 and ditriazene 10 as Sandmeyer precursors to 2.
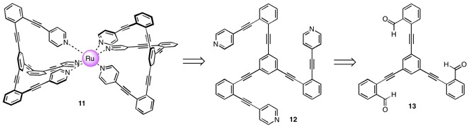
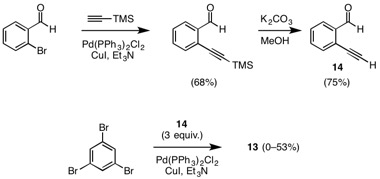
We have also progressed toward a molecule that maintains an orthogonal relationship between the central benzene ring and peripheral ¹-systems. In our initial strategy, we have attempted to generate complex 11, which comprises a central, octahedrally coordinating Ru(II) ion complexing two highly unusual pyridine-based ligands 12. Retrosynthetic analysis of 12 disconnects to trialdehyde 13 via Corey–Fuchs and Sonogashira methodologies.
Our synthesis begins with a Sonogashira cross-coupling of TMS-acetylene and 2-bromobenzaldehyde and subsequent deprotection, which successfully affords 2-ethynylbenzaldehyde (14). Sonogashira coupling of 14 with 1,3,5-tribromobenzene has produced 13 in reasonable yields, but reproducibility of this reaction has been an issue.
This award has been instrumental at the beginning of my independent career by supporting high-quality research opportunities for undergraduate students. The award has enabled a total of eight undergraduate students to take part in these projects and has directly supported four students over the summer months. We look forward to showcasing work supported by this award in conferences in the coming year.