Reports: ND655183-ND6: Electronic Spectroscopy of Organic and Organometallic Ions
Michael D. Morse, University of Utah
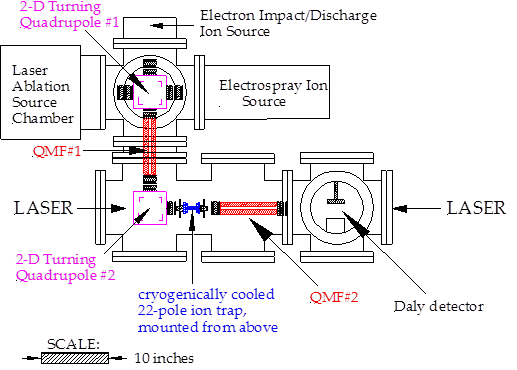
Funding for this grant began on January 1, 2015 and was used primarily to provide salary for my postdoctoral researcher, Sergei Aksyonov, who worked to complete the construction of the ion trap instrument. The schematic design of the instrument is shown below:
Sadly, Sergei died very suddenly and unexpectedly on September 11, 2015, before this instrument became fully operational, and at that point all progress ceased abruptly. Prior to his death, Sergei had repaired the electronics used to drive the quadrupole mass filters QMF#1 and QMF#2, and these are now working well, providing nicely resolved mass spectra. The turning quadrupole, TQ#2, has been optimized to turn the ion beam 90º while maintaining low kinetic energies (in the range of 5-10 eV). We have successfully cooled the ion trap down to 4.5K in the absence of helium collision gas, and to 10K with helium collision gas present. Most importantly, the heart of the instrument, the 22-pole trap, has been used successfully to trap low-energy ions. We have been able to trap approximately 30,000 ions, which can be held in the trap for many minutes, but this required an accumulation time of about 5 minutes. Sergei was in the process of sorting out why the rate of ion trapping is so much slower than expected when he passed away. I have now recruited a very competent graduate student, Jason Sorensen to work on this project, and we have begun to improve the instrument by purchasing a commercial electron impact ionizer to use as our ion source.
The heart of the instrument, the 22-pole ion trap, is completed, and we have been able to trap roughly 3400 FeCO+ ions formed by electron impact ionization/dissociation of Fe(CO)5. This was achieved using to homemade electron-impact ion source. We anticipate greater ion currents using the commercial device. To complete the instrument, we need to determine why our trapping efficiency is low, add the laser ablation source, and set up the photodissociation laser source. With the laser ablation ion source, we expect to produce several orders of magnitude more ions than can be produced with the electron impact source, allowing far greater numbers of ions to be trapped. This will also greatly increase the diversity of species that can be studied as compared to the electron impact ionization source, and will be needed for the studies of metal-methane reaction products.
When the construction of the instrument is complete, our first targets for study will be generated by electron impact of organic molecules, and will consist of protonated benzene, allyl cation, pentadienyl cation, heptatrienyl cation, and acylium ions.