Reports: ND155514-ND1: Microwave-Promoted Tin-Free and Initiator-Free Cyclizations of Iminyl and Aminyl Radicals: A Convenient New Method of Synthesizing Complex Amines from Simple Precursors
Steven L. Castle, Brigham Young University
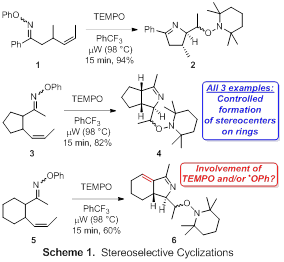
During the first year of this PRF-funded project, our efforts to discover new microwave-promoted iminyl radical cyclizations (Aim 1) have focused on two areas. We have investigated stereoselective cyclizations, and we have explored the use of radical traps other than TEMPO to facilitate the formation of carbon-carbon or carbon-heteroatom bonds. Our work in pursuit of the former objective is summarized in Scheme 1. Subjection of O-phenyl oximes 1, 3, and 5 to microwave irradiation in the presence of TEMPO with trifluorotoluene as solvent afforded dihydropyrroles 2, 4, and 6 in good to excellent yields. The negligible stereocontrol with respect to the exocyclic stereocenters (ca. 1.0–1.5 dr) was anticipated. Fortunately, in each case the stereocenters on the newly formed dihydropyrrole rings were generated with complete control. The dehydrogenation that occurred in the cyclization of cyclohexane-containing substrate 5 was unexpected, so we verified this result by successfully repeating the reaction. Interestingly, no dehydrogenation was observed in the cyclization of cyclopentane-containing substrate 3. We speculate that a TEMPO- or phenoxy-radical-mediated hydrogen atom abstraction might be mediating the dehydrogenation of 5. The failure of 3 to follow a similar pathway may be due to the increased ring strain caused by forming a radical in a 5-membered ring versus in a 6-membered ring. We will investigate other potential substrates to determine the scope of this unusual cyclization/dehydrogenation process.
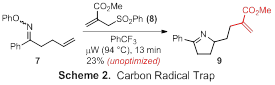
While our studies of alternative radical traps are less advanced at the current time, we have performed a single cyclization with a carbon radical trap. Although optimization is needed, microwave irradiation of O-phenyl oxime 7 in the presence of allylsulfone acrylate 8 furnishes dihydropyrrole 9 as the product of a tandem C-N/C-C bond-forming process (Scheme 2). We are encouraged by these results and will conduct a detailed investigation of microwave-promoted iminyl radical cyclizations using other radical traps in Year 2 of the project.
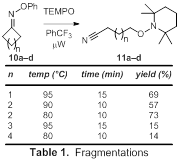
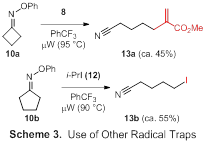
Our attempts to perform aminyl radical cyclizations have been characterized by reactions yielding complex mixtures that were challenging to interpret. Accordingly, we elected to shift the focus of Aim 2 by exploring novel transformations of iminyl radicals (i.e., processes that do not involve cyclizations). Excitingly, we learned that cyclic oxime ethers 10a-d undergo fragmentations upon microwave irradiation, delivering difunctionalized adducts 11a-d upon trapping of the acyclic radicals with TEMPO (Table 1). Fragmentations of 10a (n = 1, 4-membered ring) and 10b (n = 2, 5-membered ring) were facile, delivering difunctionalized adducts 11a and 11b in good yields. Notably, studies with 10b demonstrated that the reaction was complete in 10 min at 80 °C. Fragmentation even occurred at 70 °C, although the reaction had only proceeded to ca. 25–30% conversion after 10 min. The six-membered substrate 10c also underwent fragmentation, but only to a small extent. The low yield is presumably due to the lack of ring strain removing one of the driving forces for the process. Seven-membered substrate 10d behaved similarly to 10c, furnishing the adduct 11d in low yield. The scope of fragmentations with alternative radical traps appears promising, as initial experiments conducted on 10a with allylsulfone acrylate 8 as the radical trap as well as on 10b with i-PrI (12) produced the functionalized adducts 13a and 13b in moderate yields (Scheme 3). We are currently pursuing the use of additional radical traps capable of installing a variety of functional groups via microwave-promoted fragmentations of several different 4- and 5-membered cyclic oxime ethers.
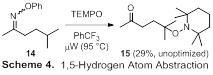
We have also explored the ability of microwave-generated iminyl radicals to engage in 1,5-hydrogen atom abstractions. Irradiation of O-phenyl oxime ether 14 in the presence of TEMPO afforded ketone 15 in modest yield (Scheme 4). Interestingly, products derived from hydrogen atom abstraction and TEMPO trapping at the methylene carbons, including di-TEMPO adducts, were also obtained. This indicates that intermolecular hydrogen atom abstraction is competitive with the intramolecular process and provides clues for optimizing this transformation. Since the immediate precursor to oxime ether 13 is a ketone, its two-step conversion into ketone 14 constitutes a formal g-C–H functionalization. This is significant, as the g-position of a ketone is quite difficult to functionalize. Although the yield has room for improvement, we are excited about this result and plan to optimize the reaction during Year 2 of the project.
Three students have worked on this project: MS student Mary Jackman, undergraduate Siyeon Im, and undergraduate Seth Bohman. Mary is planning on graduating and applying to Ph.D. programs next year, and the PRF support she has received is critical to enabling her to achieve an impressive body of research prior to graduation. Siyeon will also graduate next year, and her experience working on this project has inspired her to apply to Ph.D. programs in chemistry. Seth is interested in applying to medical school upon graduation, but his research experience has been very positive and sparked an interest in performing research as a clinician. In addition to positively impacting these students, the PRF funding has been crucial to my own career. The results described above that we were able to obtain with PRF support have formed the basis of an NSF proposal that we submitted this week with the intent of securing funding for this project when the PRF award expires next year. Without PRF support, our preliminary results would have been much less impressive and our chances of receiving an NSF grant would be slim. Thus, I am very grateful that the ACS PRF was willing to fund a New Direction in my research at this time in my career.