Reports: DNI354413-DNI3: Development of Sustainable Co(I) Catalyst Toward C-N Bond Coupling
Alison R. Fout, PhD, University of Illinois at Urbana-Champaign
The catalytic formation of C-C bonds via transition metal catalyzed cross-couplings is an important and well-known area of Organometallic chemistry. The coupling of Csp2-Csp2 bonds has been extensively developed and mechanistic studies gave insight into the reaction. Despite the important of these reactions in the pharmaceutical and fine chemical industries, these reaction motifs are not well-suited for the formation of Csp2-Csp3 bonds. We were particularly interested in targeting the formation of highly encumbered C-C bonds via Kumada coupling and a cobalt catalyst. Based on our work of the cobalt catalyzed C-N bond formation and mechanistic insights obtained, we hypothesized that the various phosphine ligated cobalt catalysts previously synthesized in our group should be amendable to such chemistry.
Our initial studies centered on the cross-coupling of neopentyl magnesium bromide or tert-butyl magnesium chloride to aryl iodides. Three different cobalt catalysts were screened (which were described in the 2016 progress report), (PPh3)3CoN(SiMe3)2, (dppf)CoN(SiMe3)2, and (DPEPhos)CoN(SiMe3)2. After some optimization the best yield obtained with any of the catalysts was 35%. While the cross-coupling of these reagents is quite difficult, the low yield in the reaction prompted us to screen various cobalt catalysts. In particular, we targeted coordinately unsaturated cobalt(I) catalysts. In doing so, we targeted a similar reaction sequence reported by Oshima and Cahiez, in which an uncharacterized Co(I) species is generated from the reaction of Co(acac)3 (acac = acetylacetonate) and excess alkyl or aryl Grignard reagents in the presence of bidentate phosphines or amine ligands.
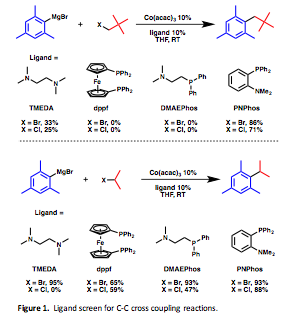
Co(acac)3 was dissolved in THF and neopentyl bromide, and mesitylmagnesium bromide were added in the presence of various bidentate ligands and an immediate color change was observed. The results of the ligand screen for the cross-coupling reaction are summarized in Figure 1.
As shown, TMEDA and dppf gave good yields for the cross-coupling of mesitylmagnesium bromide and isopropylbromide, but low yields, in the case of TMEDA, for the more bulky neopentylbromide and no products were isolated when dppf was used. To bridge the gap in the reactivity observed a hybrid amine/phosphine ligand was chosen, DMAEPhos. This ligand demonstrated reactivity comparable to TMEDA. However, PNPhos proved to be remarkably effective at the coupling of neopentylhalide with mesitylmagnesium bromide – a result we had only observed in modest yields with TMEDA as the ligand.
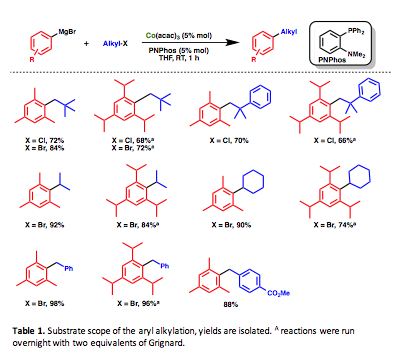
Further exploration into this reactivity yielded the formation of 12 new highly encumbered cross-coupled products which are summarized in Table 1. The reactions are remarkably fast (in as little as 40 minutes) and occur at room temperature. The reactions are largely air- and moisture-stable and can be carried out either in a glovebox or under nitrogen on a Schlenk line. Notable reactions include the coupling of 2,4,6-triisopropyphenylmagnesium bromide as well as the formation of neophylated products.
In an effort to understand the functional group tolerance, a tolerance screen was carried out in a manner described by Glorius and coworkers, where a reaction can be screened by using functional group bearing additives rather than substrates with intrinsic functionality. A high-yielding reaction was targeted and various additives were chosen to gauge the tolerance of a various of functional groups. From this we established that protic and enolizable functionality hinder the reaction, but can tolerate methyl esters and acetamide. Pyridines, benzonitriles are also tolerated but dimethylaniline inhibits the reaction with loss of nearly 50% of the product and olefins hinder the reaction likely do to binding to the metal center.
Currently, we are targeting a detailed mechanistic study of this reaction. We have been targeting and trying to isolate the active catalyst of this system, as we propose that is likely a highly-reactive (PNPhos)Co(I) alkyl complex. Thus far, isolation of the active catalyst has remained elusive and targeting similar molecules has not been effective.
We have developed a new method for the preparation of highly encumbered C-C bonds. Using an in situ generated cobalt catalyst featuring an aminophosphine ligand, PNPhos, we have been able to install neopentyl and neophyl groups on sterically crowded arylmagnesium halides. This method is quite simple operating under mild conditions and in short times without the need to rigorously exclude air and moisture. While the mechanistic understanding of this reaction is ongoing, the remarkable reactivity has great potential.
Three students have worked on various aspects of this project have been able to present their research findings at National ACS meetings and network with scientists from around the world. The results of this project have been disseminated at conference proceedings and will be published upon completion of the mechanistic study.