Reports: DNI155704-DNI1: Catalyst-Directed C-H Functionalization for the Streamlined Use of Petroleum Feedstocks
David A. Nagib, PhD, Ohio State University
Excellent progress has been made toward our goal of developing new methods to elaborate petroleum feedstock chemicals via targeted C-H functionalization. For example, in a recently published article, we described the development of a new protocol for the δ C-H amination of unactivated, secondary C-H bonds to form a broad range of functionalized pyrrolidines. To successfully achieve this goal, we developed a new triiodide-mediated activation strategy, in which selective hydrogen atom abstraction of unbiased C-H bonds could be achieved via in situ generation and sequestration of transiently generated iodine. Through oxidation of sodium iodide (NaI), the reactive iodine (I2) species could be controllably synthesized, while excess reagent is also trapped as triiodide (I3−). This triiodide-based strategy has significantly expanded the synthetic utility of the classic Hofmann-Löffler-Freytag (HLF) reaction and now enables access to a range of complex, biologically relevant pyrrolidine molecules from much simpler, petroleum-derived feedstocks.
Progress
Below is an outline of the specific progress that we have made towards the first two specific aims outlined for this project.
Triiodide-Mediated δ-Amination of Secondary C-H Bonds
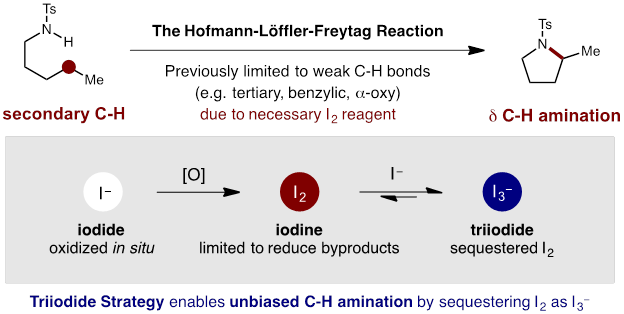
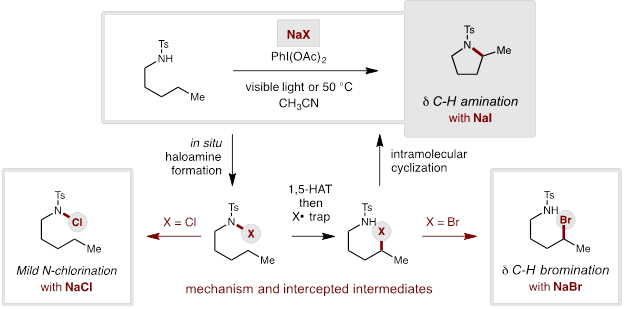
A key aspect of our strategy to develop new synthetic methods for targeted C-H functionalization of petroleum feedstock chemicals is the classic Hofmann-Löffler-Freytag (HLF) reaction. Building on its enabling potential, we addressed a major limitation in which the C-H bonds must be biased or activated (e.g. tertiary, benzylic, α-oxy). To extend the synthetic scope to include unbiased, secondary δ C-H bonds, we sought to limit the concentration of iodine (I2) and in situgenerated acetyl hypoiodite (AcOI), which are prone to photo-initiated homolysis that leads to undesired oxidations of weaker C-H bonds.
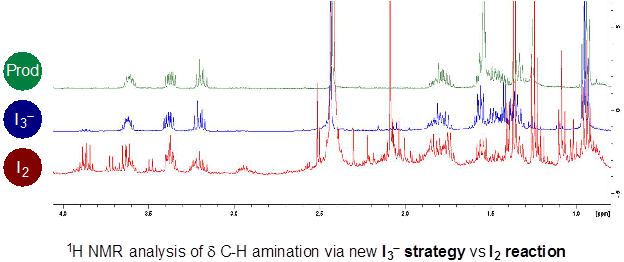
Our solution to the I2-problem entails slow, in situ I2-generation from innocuous iodide (I−) salts. This strategy allows for (i) attenuated formation of transiently reactive AcOI, and (ii) decreased generation of byproducts via in situ sequestration of I2 as triiodide (I3−). This approach precludes undesired I2-mediated decomposition that can otherwise limit synthetic utility to only weak C-H bonds. The synthetic value of this triiodide strategy is evident in the NMR spectroscopic analysis of the crude reactions from the I2 vs I3−based strategies.
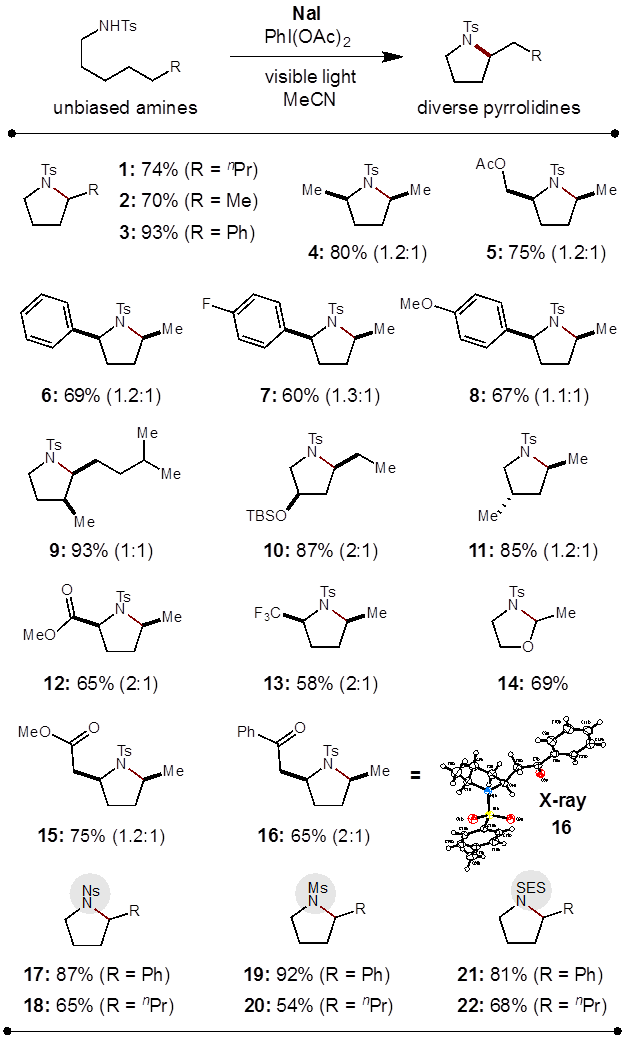
This approach has now enabled the δ C-H amination of unactivated, secondary C-H bonds to form a broad range of functionalized pyrrolidines. Among the many amines that successfully undergo this transformation, we have observed a wide substrate tolerance for synthetically useful functionality, including ethers, esters, ketones, and organofluorines.
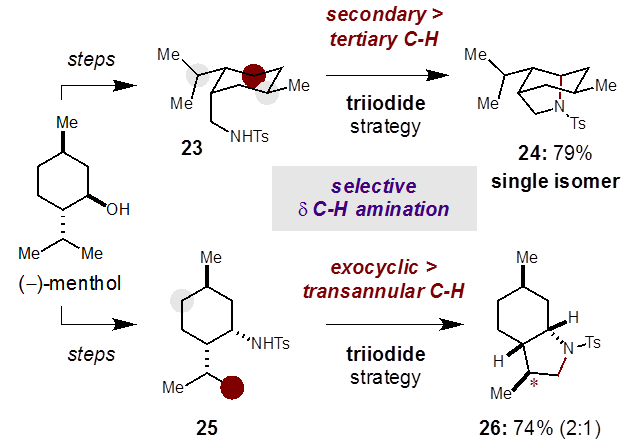
Notably, this strategy for the δ C-H amination exhibits atypical selectivity for secondary C-H bonds, even in the presence of weaker, tertiary C-H bonds.
Interestingly, the mild polyhalide reaction conditions are readily extended to other halide systems by simply switching from NaI (triiodide) to NaBr or NaCl (poly -bromide or -chloride). In these latter cases, subjecting simple amines to such poly-halide activation enables the isolation of two postulated intermediates from the HLF mechanism.
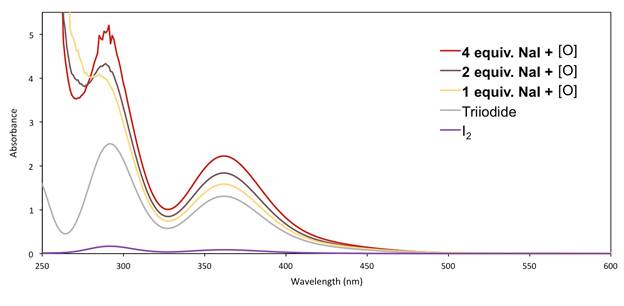
Further support for the role and presence of triiodide in our mechanism is found in the observation of the I3−species by UV-Vis spectroscopy, including under relevant reaction conditions.
Impact
The successful completion and publication of this ACS PRF-funded research has had a major impact on the establishment of our research lab as well as the effective training of personnel who were supported by this grant, including one undergraduate student, two graduate students, and a post-doctoral fellow. Our dissemination of this research has garnered positive attention in the synthetic organic and C-H functionalization communities, through fruitful interactions at three conferences (regional, national, and international), and as illustrated by a recent in-depth highlight of our work in the journal, Synform. Importantly, based on preliminary results obtained through this grant, our lab was recently awarded an R35 from the National Institutes of Health (NIH).