Reports: UNI653818-UNI6: Extending the Applications of SALDI-MS for Asphaltene Analysis Using Transition Metal Oxide Nanopowders
Karen Virginia Sinclair Molek, PhD, University of West Florida
PROJECT OBJECTIVES: The purpose of this research is to extend solvent-free investigations using transition metal oxide (TMO) nanoparticles as surfaces for the “soft” ionization/desorption of asphaltenes and petroleum products using surface-assisted laser desorption/ionization mass spectrometry (SALDI-MS). Accessibility of instrumentation capable of asphaltene detection and determination of petroleum composition and properties via MS are invaluable, particularly during times of high demand such as the 2010 BP Horizon Oil Spill. The current asphaltene analysis research extends previous “capture and removal” of asphaltene studies, viaTMO nanoparticles, to investigate the utility of TMO nanoparticles as surfaces in SALDI-MS.
RESEARCH PROGRESS:
The TMO nanoparticles (NiO, Fe3O4, Co3O4, and TiO2) utilized for asphaltene adsorption and as surfaces for SALDI were commercially obtained but characterized in-house. Asphaltene samples were donated by the National High Magnetic Field Laboratory at Florida State University and were extracted with n-heptane and fractionated according to IP 143/90 method.
- A. Nanoparticle Characterization
The textural properties (particle size and specific surface area (SSA)) of the nanoparticles reported herein are listed in Table 1 and the particle sizes are within the expected nanometer scale.
- B. Asphaltene Adsorption
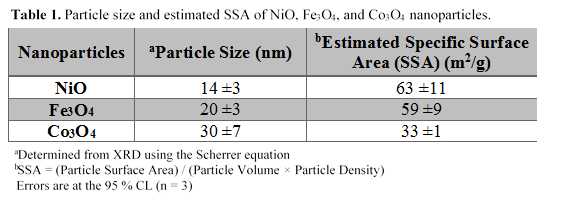
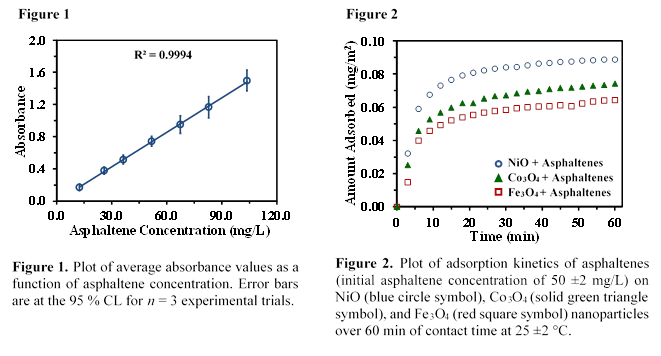
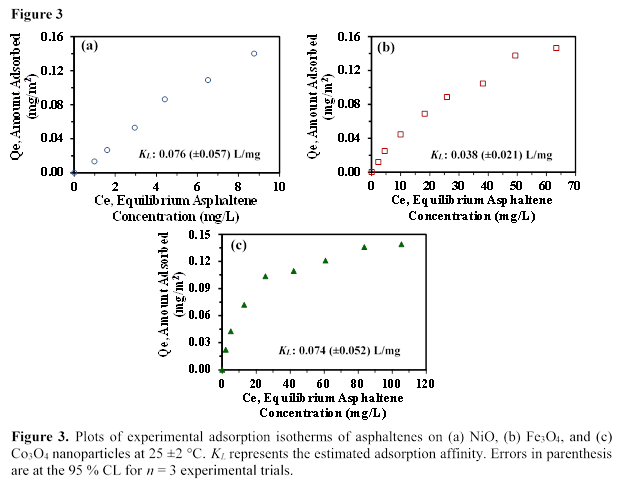
We carried out asphaltene adsorption on NiO, Fe3O4, and Co3O4 nanoparticles and we determined the concentration of unknown asphaltenes using the calibration curve presented in Figure 1. The calibration curve was obtained from the UV-Visible absorbance of asphaltenes at 375 nm within asphaltene concentration range of 12 to 104 mg/L. Figure 2 represents the adsorption kinetics of asphaltenes conducted at room temperature (25 ±2 °C) over a 60-min period. The adsorption rate shows rapid adsorption of asphaltenes (i.e., equilibrium was achieved within 60 mins of contact time between the asphaltenes and the nanoparticles) demonstrating the non-porosity of the nanoparticles for efficient adsorption of asphaltene molecules. The slopes from the linear portions of the asphaltene adsorption kinetics plots of NiO, Fe3O4, and Co3O4 were determined to be 1.6 (±0.1) × 10-4 mg/m2s, and 1.1 (±0.2) × 10-4 mg/m2s, and 1.3 (±0.1) × 10-4 mg/m2s, respectively. Similarly, we estimated the adsorption affinities of asphaltenes on NiO, Fe3O4, and Co3O4, nanoparticles for a monolayer coverage using the Langmuir adsorption model. Figure 3 shows the experimental adsorption isotherms for NiO, Fe3O4, and Co3O4 nanoparticles conducted at room temperature using an initial asphaltene concentration range of 9 to 149 (±1) mg/L. Figures 3(a-c) show that NiO nanoparticles have a higher adsorption affinity (KL) than Fe3O4 and Co3O4nanoparticles towards asphaltene molecules, indicating a stronger interaction with the asphaltene molecules.
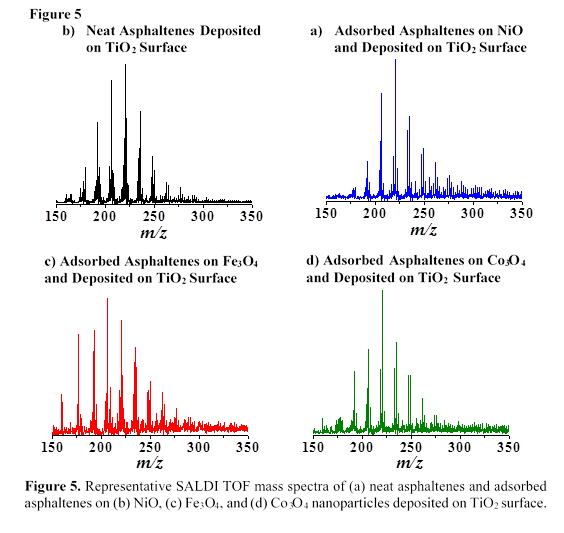
C. SALDI MS of Asphaltenes Using TMO Nanoparticles
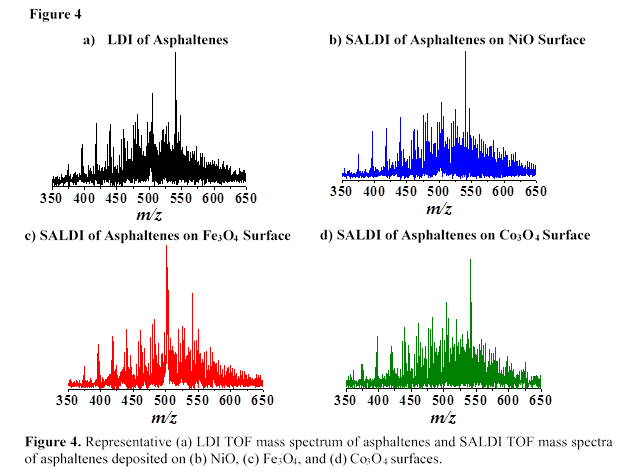
NiO, Fe3O4, Co3O4, and TiO2 nanoparticles were used as surfaces for SALDI MS analysis of asphaltenes. Figure 4 shows representative LDI and SALDI TOF mass spectra of neat asphaltenes for the m/z range of 350 to 650 Da. A comparison of the SALDI TOF mass spectra in Figures 4(b-d) to the LDI TOF mass spectrum in Figure 4(a) reveals similar asphaltene mass distribution patterns. Most of the ionic species observed in the mass spectra of asphaltenes are nanoaggregates and fragment ions due to the simultaneous incidence of desorption and ionization processes during SALDI. The MS results in Figure 4 suggest that TMO nanoparticles efficiently desorb and ionize asphaltene molecules using the SALDI method.
In Figure 5, we utilized TiO2 as SALDI surface and observed similar asphaltene mass distribution in neat asphaltenes (Figure 5(a)) and adsorbed asphaltenes (Figures 5(b-d)) which demonstrate the effectiveness of NiO, Fe3O4, and Co3O4 nanoparticles as adsorbents for asphaltene molecules. In addition, we did not observe matrix effects at low m/z range (i.e., m/z <350 Da) using TMO nanoparticles as SALDI surfaces for MS analyses of asphaltenes.
FUTURE EXPERIMENTS:
We plan to study asphaltene adsorption using TMO films and quantum dots. In addition, we will utilize a high resolving power MS instrument such as an FT-ICR mass spectrometer to analyze asphaltene molecules adsorbed on various TMO nanoparticles.
STUDENT PARTICIPATION, PUBLICATIONS AND PRESENTATIONS:
A total of seventeen undergraduate chemistry majors have worked on this project, each for 3 – 8 semesters. Of the total, 6 have graduated with 5 currently enrolled in a Chemistry PhD program and 1 enrolled in a professional Master’s of Science program. An additional two students will graduate in May 2017, both of whom are applying to PhD programs and I am confident will be accepted.
Five of the undergraduate students were supported via grant funds and nine additional students were supported by the UWF Summer Undergraduate Research Program. Fourteen of the undergraduate students have presented 17 poster presentations at the following conferences/symposia: two at the Spring 2014 National ACS meeting (one was invited to participate in Sci-Mix), two at the Spring 2015 National ACS meeting, two at the Spring 2016 National ACS meeting, four total at the 2014, 2015, and 2016 Annual Biomedical Conference for Minority Students (ABRCMS), and seven at the 2014, 2015, and 2016 UWF Student Scholars Symposium (one Best STEM Poster and one Best Chemistry Poster). Grant travel funding was used to support the six ACS and four ABRCMS presentations. A manuscript has been submitted to the ACS Journal Energy and Fuels detailing the SALD-MS results. Four additional students have submitted abstracts to present the recent results at the Spring 2017 National ACS meeting. Additionally, UWF hired Dr. Abayomi Daniel Olaitan in February 2016 to help run my research group and work on the PRF funded grant. Dr. Olaitan has been invaluable in both helping train additional students and expediting research results. He will be presenting a research presentation at the 2017 ASMS.