Reports: ND454555-ND4: Photochemistry in Liquid Crystalline Media - A Novel Way to Control Selectivity of Photoreactions
Evgueni E. Nesterov, Louisiana State University
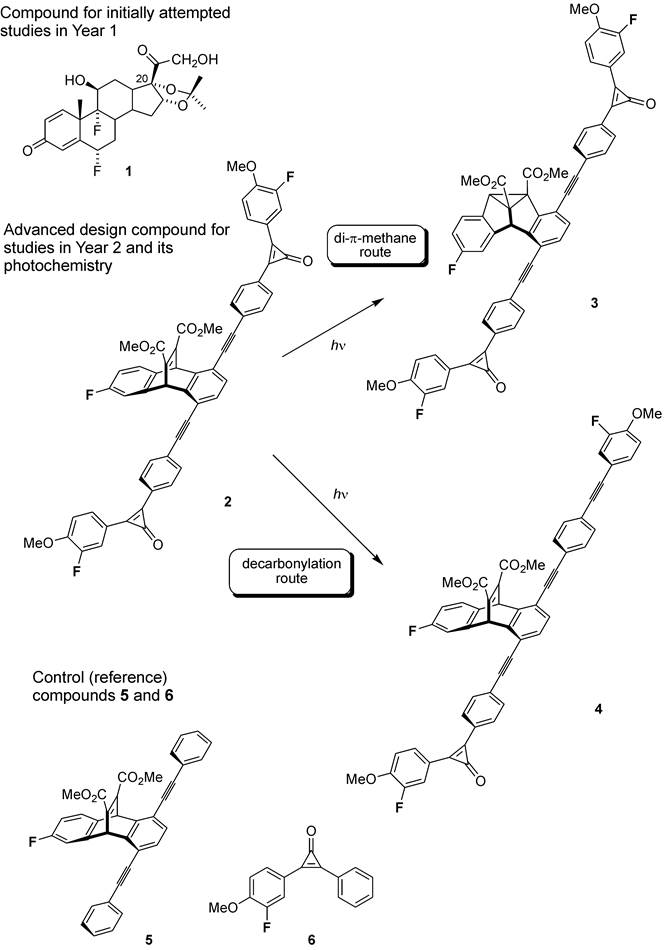
The ultimate goal of this project is to develop a fundamental approach to increase and control selectivity of photochemical reactions using spatially selective excitation of photoreactive compounds in liquid crystalline (LC) host media. As a working strategy, we study nematic LC media to impose a unidirectional orientation on an array of dissolved photoreactive guest molecules. Upon achieving the uniform orientation of the guest molecules, the LC solution is irradiated with plane polarized light in order to selectively excite a particular electronic transition in the guest molecule, which is expected to lead to an increased selectivity of their photochemical transformations. In the first year of the project, we carried out two main tasks. First, we designed and built a functional photochemical setup to carry out spatially selective photoirradiation of LC solutions loaded in custom-made LC aligning cells (it was described in more detail in the first report). The second task was to optimize preparation of oriented nematic LC samples, and carry out photochemical studies with a commercially available compound 1. Compound 1 possessed two photochemically reactive independent fragments: conjugated dienone moiety in ring A reacting via type A enone (“lumiketone”) rearrangement, and the ketone group at C20 to yield Norrish type I photocleavage. Although variable angle photoirradiation of the oriented 1-LC thin samples did demonstrate some variation of the two products ratio with the light incidence angle, the quantitative results were not reproducible. We assigned these problems to insufficient alignment of compound 1 by nematic LC 5CB, as well as poor ability of the nematic LC phase to “hold” the molecule 1 in a fixed position. Thus, in the second year we spent majority of experimental efforts on synthesis and study of the new photoreactive compound 2 of rigid rod shape, which was designed to show much better alignment in the nematic LC host matrix. The compound 2 is based on dibenzobarrelene (triptycene) scaffold, which can undergo efficient di-p-methane photorearrangement on the triplet (T1) potential energy surface. The triptycene scaffold provides an efficient “handle” for alignment of 2 in the nematic LC phase due to the “free volume” effect. In agreement with the structural design, compound 2 aligned well in 5CB medium which was confirmed using polarized UV/vis spectroscopic studies (the absorption anisotropy of ~0.6 was experimentally found). Each dibenzobarrelene unit in 2 was linked to two diphenylcyclopropenone units – which have been well documented to undergo efficient photochemical fragmentation on the S2 surface with CO release and formation of diphenylacetylene. The design of 2 also assumed that the two cyclopropenone carbonyl moieties would sensitize intersystem crossing of the di-p-methane dibenzobarrelene moiety to the required T1 state. Both dibenzobarrelene and diphenylcyclopropenone units were fitted with fluoro-substituents, to be used for monitoring the photoreaction by 19F NMR spectroscopy without the need to separate the reaction mixture from the LC host. Compound 2 was prepared using Sonogashira coupling of the individual building blocks. In addition to the target compound 2, we also synthesized control compounds 5 and 6, to investigate individual photochemistry, and to determine changes in fluorine signals chemicals shifts upon corresponding transformations, to be used in monitoring photoreactions of 2. Photochemical irradiation of 5 and 6 was carried out on preparative scale upon irradiation with Pyrex-filtered UV light, and both di-p-methane product from 5, and diphenylacetylene product from 6 were purified and separately characterized (to be used as references in 19F NMR spectroscopy monitoring of photoirradiation of 2).
Photoirradiation of 2 aligned in nematic LC (5CB) matrix was carried out with Pyrex-filtered plane polarized light with polarization angles varying in 10° increments from 0 to 90° relative to the LC director. The cell temperature was strictly controlled to ensure that LC remained in nematic phase, and photoirradiation was typically carried to approximately 10% conversion. The product composition was analyzed by 19F NMR after dissolving the entire sample in CDCl3. Since 19F NMR peaks corresponding to both products could be easily observed even at relatively low conversions, and without interference from 5CB matrix, this allowed reliable quantification of the results directly from the LC solution, without need to carry out tedious separations. We carried out multiple runs, with varying irradiation times, and different incident UV light intensity. We found that the ratio between the di-p-methane and decarbonylation photoproducts 3 and 4 showed strong dependence on the polarization angle. The di-p-methane rearrangement was the predominant pathway at polarization angles within 20 to 40° relative to the LC director, whereas the decarbonylation product 4 was predominantly observed at the angles of 60 and 70°. A mixture of the two products was observed at other angles. This behavior was rather reproducible, with small batch-to-batch quantitative variations. Currently, we are working on building a larger size LC cell, so the polarized light photoirradiation could be carried out at a preparative scale, in order to separate and characterize both photoproducts. This is required for confirmation of our structural assignment of photoproducts based on 19F NMR spectroscopy. In addition, we are carrying our computational studies to determine direction of transition dipole moments for each reactive unit in 2, so we could relate these to experimentally observed angular dependence of the two photoproducts. Upon completion of this work, we plan on submitting a manuscript to a high-impact journal.
In conclusion, in the second and final project year we obtained convincing and promising results which show that our proposed approach to control selectivity of photochemical reactions by spatially selective excitation does work. Upon concluding these studies, the results will be submitted as a research paper, and we are working on putting together an NSF proposal to fund continuation of this project.