Reports: DNI655481-DNI6: Elucidating Support Effects and Size Effects of the MoS2-Based Catalysts from First-Principles Calculations
Kesong Yang, PhD, University of California San Diego
In the past grant period, 01-Sep-15 to 31-Aug-16, the major research grant activity is devoted to understand size effects on the structural stability of MoS2 nanosheets, and the formation of sulfur vacancies in the two-dimensional (2D) MoS2 nanosheets using first-principles calculations. As detailed below, this grant already yielded significant research, academic, and education achievements. For example, under the (partial) support of the grant, one paper was published in the prestigious journal of Physical Chemistry Chemical Physics in the field of physical chemistry, and one paper was under preparation. Under the support of the grant, graduate student Mr. Paul Joo in the PI's research group was awarded 2016 GSA Travel Grant for MRS Spring Meetings & Exhibits, and also won the best poster award in the first Southern California Theoretical Chemistry Symposium.
2. Accomplishment of Research Activities
The research accomplishments are detailed as below:
a) Research Results, Conclusions, and Next-Step Research Plan
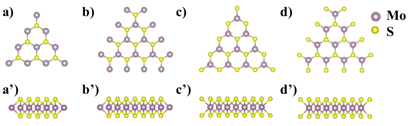
In the first year of the grant period, we focused on the computational/theoretical understanding of the size effects of 2D MoS2 nanosheet and the sulfur vacancies in these nanosheets. It is experimentally observed that the single-layer MoS2 often exist as triangle shape. Hence, in this work, we have modeled four types MoS2 edge structures with zig-zag pattern, defined as ZZ-Mo-1, ZZ-Mo-2, ZZ-S-1, and ZZ-S-2, respectively, as shown in Figure 1.
Figure 1. Single-layer MoS2 nanosheets (n=4) with zig-zag patterned edges from top view (a-d) and side view (a'-d'), respectively. (a,a') ZZ-Mo-1, (b,b') ZZ-Mo-2, (c,c') ZZ-S-1, and (d,d') ZZ-S-2. The large purple and small yellow balls represent Mo and S atoms, respectively. n standards for the number of Mo atoms in the edge of the MoS2 nanosheet.
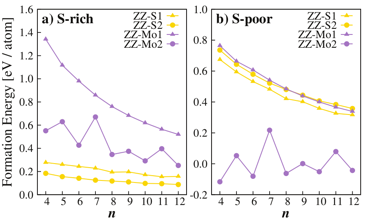
The calculated formation energies of the MoS2 nanosheets, under extremely S-rich and S-poor conditions, are displayed in Figure 2a and 2b, respectively. Under extremely S-rich condition (see Figure 2a), the S-terminated edge structures (ZZ-S-1 and ZZ-S-2), clearly, have lower formation energy than the Mo-terminated edge structures (ZZ-Mo-1 and ZZ-Mo-2). This is in an agreement with experimental results, in which S-terminated edge structures were found to be more stable. Moreover, under this condition, the ZZ-S-2 structures are always more stable than the ZZ-S-1 structures for any nanosheet size. Under extremely S-poor (Mo-rich) condition (see Figure 2b), a similar trend of the formation energy decreasing as the nanosheet size is found for ZZ-S-1, ZZ-S-2, and ZZ-Mo-1 structures. For ZZ-Mo-2 structure, interestingly, there exists an odd-even effect for the formation energy as the size of nanosheet. A systematic analysis for the behavior of these four types edge structures was carried out, and the origin for such an odd-even behavior can be explained from their relaxed geometrical structures, see Figure 3. For ZZ-S-1, ZZ-S-2, and ZZ-Mo-1 structures, upon relaxation, they exhibit similar structure characteristics for both odd- and even-size nanosheets. In contrast, for even-size ZZ-Mo-2 structure (Figure 3b), Mo atoms on the edges of the triangle nanosheet are paired with their adjacent atoms, lowering the energy of the system; for odd-size ZZ-Mo-2 structure (Figure 3b'), the nanosheet has one extra Mo atom that cannot be paired with its adjacent atoms, and thus the three Mo atoms at the center of the edges are left unpaired, which exhibit relatively higher formation energy than that of even-size nanosheets.
Figure 2. Calculated formation energies for the relaxed four types of MoS2 nanosheets (ZZ-S-1, ZZ-S-2, ZZ-Mo-1, and ZZ-Mo-2), under (a) extremely S-rich and (b) S-poor conditions, with respect to n, the size of the nanosheets.
We next plan to explore the formation of sulfur vacancies in the MoS2 nanosheets. This is because sulfur vacancies play a major role in the hydrodesulfurization catalytic process of MoS2 catalysts. Some interesting preliminary results were already obtained. For sulfur-terminated structure ZZ-S-2, there exists a clear odd-even pattern for the sulfur vacancy formation: for the sulfur vacancy at corner site, the formation energy of sulfur vacancy in an odd-size MoS2 nanosheet is much lower than that for an even-size nanosheet; while for the sulfur vacancy at the center of the edge, the formation energy of sulfur vacancy in an even-size MoS2 nanosheet is much lower than that for an odd-size nanosheet. A systematic and comprehensive analysis is being carried out.
Figure 3. Relaxed geometrical structures of the four types MoS2 nanosheets: (a,a') ZZ-Mo-1, (b,b') ZZ-Mo-2, (c,c') ZZ-S-1, and (d,d') ZZ-S-2. The upper and bottom panels correspond to nanosheet size n = 6 and n=7, respectively.
b) Research Achievements
The research project under the grant has a significant positive impact on the PI's academic career. The grant helps further establish the PI's research group, and expand the PI's research direction in the materials applications of petroleum industry. One important goal of this project is to develop a scientific workforce to the petroleum-related research. Hence, the grant also significantly influences the students (graduate student Mr. Paul Joo, Mr. Jianli Cheng, and undergraduate student Miss Camille Bernal) by providing them research opportunities and introducing them the frontiers of petroleum research.
i) Original Peer-Reviewed Work Produced by the Grant
Paul H. Joo, Maziar Behtash, and Kesong Yang*, Energetic Stability, Oxidation States, and Electronic Structure of Bi-doped NaTaO3: First-Principles Hybrid Functional Study, Phys. Chem. Chem. Phys. 18, 857-865, (2016).
Paul H. Joo, Jianli Cheng, and Kesong Yang*, Odd-Even Effects in the Structural Formation of Two-dimensional MoS2 Nanosheet: First-Principles Study, (In Preparation)
ii) Students' Research Award Produced by the Grant
Paul Joo, 2016, GSA Travel Grant for MRS Spring Meetings & Exhibits
Paul Joo, 2016, Best Poster Award, First Southern California Theoretical Chemistry Symposium
iii) Abstract Presented in Conference Produced by the Grant
2016 MRS Spring Meetings & Exhibits, Phoenix, AZ. Paul Joo, Mazir Behtash, and Kesong Yang, Energetic Stability, Oxidation States, and Electronic Structure of Bi-doped NaTaO3: First-Principles Hybrid Functional Study.